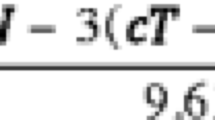Abstract
Sargent et al (J Clin Oncol 23: 8664–8670, 2005) concluded that 3-year disease-free survival (DFS) can be considered a valid surrogate (replacement) endpoint for 5-year overall survival (OS) in clinical trials of adjuvant chemotherapy for colorectal cancer. We address the question whether the conclusion holds for trials involving other classes of treatments than those considered by Sargent et al. Additionally, we assess if the 3-year cutpoint is an optimal one. To this aim, we investigate whether the results reported by Sargent et al. could have been used to predict treatment effects in three centrally randomized adjuvant colorectal cancer trials performed by the Japanese Foundation for Multidisciplinary Treatment for Cancer (JFMTC) (Sakamoto et al. J Clin Oncol 22:484–492, 2004). Our analysis supports the conclusion of Sargent et al. and shows that using DFS at 2 or 3 years would be the best option for the prediction of OS at 5 years.
Similar content being viewed by others
References
Biomarkers Definitions Working Group (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69: 89–95
Burzykowski T and Buyse M (2006). Surrogate threshold effect: an alternative measure for meta-analytic surrogate endpoint validation. Pharm Stat 5: 173–186
Burzykowski T and Cortiñas Abrahantes J (2005). Validation in case of two failure-time endpoints. In: Burzykowski, T, Molenberghs, G and Buyse, M (eds) Evaluation of Surrogate Endpoints, Springer, New York
Burzykowski T, Molenberghs G, Buyse M, Geys H and Renard D (2001). Validation of surrogate endpoints in multiple randomised clinical trials with failure-time endpoints. J Roy Stat Society C (Appl Stat) 50: 405–422
Buyse M, Molenberghs G, Burzykowski T, Renard D and Geys H (2000). The validation of surrogate endpoints in meta-analyses of randomised experiments. Biostatistics 1: 49–68
Fuller WA (1987). Measurement error models. Wiley, New York
Plackett RL (1965). A class of bivariate distributions. J Am Stat Assoc 60: 516–522
Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, Piedbois P for the Meta-Analysis Group of the Japanese Society for Cancer of the Colon and Rectum and the Meta-Analysis Group in Cancer (2004) Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol 22:484–492
Sargent D, Wieand S and Haller DG et al. (2005). Disease-free survival (DFS) vs. overall survival (OS) as a primary endpoint for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23: 8664–8670
Sargent DJ for the ACCENT Group (2007) Time-dependent patterns of failure and treatment benefit from adjuvant therapy for resectable colon cancer: lessons from the 20,800 patient ACCENT dataset. 2007 Gastrointestinal Cancers Symposium, Abstract #274
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burzykowski, T., Buyse, M., Yothers, G. et al. Exploring and validating surrogate endpoints in colorectal cancer. Lifetime Data Anal 14, 54–64 (2008). https://doi.org/10.1007/s10985-007-9079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10985-007-9079-4