Abstract
Context
Habitat edges are integral features of conservation corridors and can influence corridor function and effectiveness. Edge orientation is linked to corridor design and can shape edge responses by changing habitat conditions along edges as well as contrast between conserved habitats and transformed areas.
Objectives
We assess whether corridor orientation affects butterfly assemblages in conservation corridors. To do this, we investigate how edge orientation influences butterfly diversity and abundance along forestry plantation edges, and compare this to another important design variable, corridor width.
Methods
Butterflies were recorded along the sunny austral north- and shady austral south-orientated edges in grassland conservation corridors that dissect forestry plantations, as well as corridor interior sites. Species richness, abundance and similarity to interior sites were modelled using local habitat variables (ambient temperature, floral resources, and time of day), as well as corridor design variables (corridor width, orientation and an estimate of edge contrast influenced by orientation).
Results
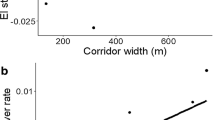
Both edge orientation and corridor width were important for butterfly diversity along corridor edges. Wider corridors enhanced overall species richness and promoted similarity between edge and interior habitats. Concurrently, grassland specialist species preferred the sunnier edges (i.e., north facing in the southern hemisphere) while forest- specialists showed a preference for the shadier edges (south facing edges). Edge orientation influenced resident butterflies more strongly than transient butterflies and influenced specialists more strongly than generalists.
Conclusions
Corridor orientation and width are complementary design variables for butterfly conservation. Wide corridors at a variety of orientations benefit different subsets of the butterfly assemblage, and the whole corridor (including both edges) is important to consider in conservation planning to capture all biodiversity.



Similar content being viewed by others
References
Aragón G, Abuja L, Belinchón R, Martínez I (2015) Edge type determines the intensity of forest edge effect on epiphytic communities. Eur J For Res 134:443–451
Arroyo-Rodríguez V, Saldaña-Vázquez RA, Fahrig L, Santos BA (2017) Does forest fragmentation cause an increase in forest temperature? Ecol Res 32:81–88
Barrios JA, De Aquino M, Di Liberto JF, Melnick D (2018) Cutting-edge meadows: The effect of meadow edges and orientation on plant community dynamics. CEC Res. https://doi.org/10.21973/N3FQ0K
Barton K (2020) MuMIn: Multi-Model Inference. R package version 1.43.17
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143
Baselga A, Orme D, Sebastien V et al (2021) betapart: Partitioning beta diversity into turnover and nestedness components. R package version 1.5.4
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models Using lme4. J Stat Softw 67:1–48
Beier P, Majka DR, Spencer WD (2008) Forks in the road: choices in procedures for designing wildland linkages. Conserv Biol 22:836–851
Bernaschini ML, Trumper E, Valladares G, Salvo A (2019) Are all edges equal? Microclimatic conditions, geographical orientation and biological implications in a fragmented forest. Agric Ecosyst Environ 280:142–151
Bjornstad ON (2020) ncf: Spatial covariance functions. R package version 1.2-9
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cardoso P, Mammola S, Rigal F, Carvalho J (2021) BAT: Biodiversity Assessment Tools. R package version 2.7.1
Chen J, Franklin JF, Spies TA (1995) Growing-season microclimatic gradients from clearcut edges into old-growth Douglas-fir forests. Ecol Appl 5:74–86
da Silva JLG, de Holanda Silva IL, Ribeiro-Neto JD, et al (2018) Forest edge orientation influences leaf-cutting ant abundance and plant drought stress in the Brazilian Atlantic forest. Agric For Entomol 20:358–365
De Smedt P, Baeten L, Proesmans W, et al (2019) Strength of forest edge effects on litter-dwelling macro‐arthropods across Europe is influenced by forest age and edge properties. Divers Distrib 25:963–974
Demaynadier PG, Hunter MLJ (1998) Effects of silvicultural edges on the distribution and abundance of amphibians in Maine. Conserv Biol 12:340–352
Dennis RH (2004) Landform resources for territorial nettle–feeding Nymphalid butterflies: biases at different spatial scales. Anim Biodivers Conserv 27:37–45
Dennis RLH, Hardy PB (2007) Support for mending the matrix: resource seeking by butterflies in apparent non-resource zones. J Insect Conserv 11:157–168
Dennis RLH, Sparks TH (2006) When is a habitat not a habitat? Dramatic resource use changes under differing weather conditions for the butterfly Plebejus argus. Biol Conserv 129:291–301
Dennis RLH, Shreeve TG, Van Dyck H (2013) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102:417–426
Dover J, Settele J (2009) The influences of landscape structure on butterfly distribution and movement: a review. J Insect Conserv 13:3–27
Dover JW, Sparks TH, Greatorex-Davies JN (1997) The importance of shelter for butterflies in open landscapes. J Insect Conserv. https://doi.org/10.1023/A:1018487127174
Evans LC, Sibly RM, Thorbek P, et al (2019) Integrating the influence of weather into mechanistic models of butterfly movement. Mov Ecol 7:24
Evans LC, Sibly RM, Thorbek P, et al (2020) The importance of including habitat-specific behaviour in models of butterfly movement. Oecologia 193:249–259
Fahrig L (2018) Forty years of bias in habitat fragmentation research. In: Kareiva P, Marvier M, Silliman B (eds) Effective conservation science: data not dogma. Oxford University Press, Oxford, pp 32–38
Fischer K, Fiedler K (2001) Resource-based territoriality in the butterfly Lycaena hippothoe and environmentally induced behavioural shifts. Anim Behav 61:723–732
Fletcher RJJ, Ries L, Battin J, Chalfoun AD (2007) The role of habitat area and edge in fragmented landscapes: definitively distinct or inevitably intertwined? Can J Zool 85:1017–1030
Flick T, Feagan S, Fahrig L (2012) Effects of landscape structure on butterfly species richness and abundance in agricultural landscapes in eastern Ontario, Canada. Ecosyst Environ 156:123–133
Franklin CMA, Harper KA, Clarke MJ (2021) Trends in studies of edge influence on vegetation at human created and natural forest edges across time and space. Can J For Res 51:274–282
Harper KA, Macdonald SE, Burton PJ, et al (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19:768–782
Harris LD (1988) Edge effects and conservation of biotic diversity. Conserv Biol 2:330–332
Hess GR, Fischer RA (2001) Communicating clearly about conservation corridors. Landsc Urban Plan 55:195–208
Ivanov K, Keiper J (2010) Ant (Hymenoptera: Formicidae) diversity and community composition along sharp urban forest edges. Biodivers Conserv 19:3917–3933
Kautz M, Schopf R, Ohser J (2013) The “sun-effect”: microclimatic alterations predispose forest edges to bark beetle infestations. Eur J For Res 132:453–465
Keeley ATH, Beier P, Creech T, et al (2019) Thirty years of connectivity conservation planning: an assessment of factors influencing plan implementation. Environ Res Lett 14:103001
Kleckova I, Klecka J (2016) Facing the heat: thermoregulation and behaviour of lowland species of a cold-dwelling butterfly genus, Erebia. PLoS ONE 11:e0150393
Laurance WF, Nascimento HEM, Laurance SG et al (2007) Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2:e1017
Leibold MA, Holyoak M, Mouquet N, et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Lukacs PM, Burnham KP, Anderson DR (2009) Model selection bias and Freedman’s paradox. Ann Inst Stat Math 62:117–125
Mecenero S, Ball JB, Edge DA, et al (2013) Conservation assessment of butterflies of South Africa, Lesotho and Swaziland: Red List and Atlas. Saftronics (Pty) Ltd., Johannesburg
Mucina L, Rutherford MC (eds) (2006) The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria
Nowicki P, Halecki W, Kalarus K (2013) All natural habitat edges matter equally for endangered Maculinea butterflies. J Insect Conserv 17:139–146
Öckinger E, Smith HG (2006) Landscape composition and habitat area affects butterfly species richness in semi-natural grasslands. Oecologia 149:526–534
Ohwaki A, Maeda S, Kitahara M, Nakano T (2017) Association between canopy cover openness, butterfly resources, butterfly richness and abundance along forest trails in planted and natural forests. Eur J Entomol 114:533–545
Ohwaki A, Koyanagi TF, Maeda S (2018) Evaluating forest clear-cuts as alternative grassland habitats for plants and butterflies. For Ecol Manag 430:337–345
Oksanen J, Blanchet FG, Friendly M et al (2020) vegan: Community Ecology Package. R package version 2.5-7
Pandit SN, Kolasa J, Cottenie K, et al (2009) Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90:2253–2262
Perović D, Gámez-Virués S, Börschig C, et al (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52:505–513
Peyras M, Vespa NI, Bellocq MI, Zurita GA (2013) Quantifying edge effects: the role of habitat contrast and species specialization. J Insect Conserv 17:807–820
Pryke SR, Samways MJ (2001) Width of grassland linkages for the conservation of butterflies in South African afforested areas. Biol Conserv 101:85–96
Pryke JS, Samways MJ (2012) Conservation management of complex natural forest and plantation edge effects. Landsc Ecol 27:73–85
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reino L, Beja P, Osborne PE et al (2009) Distance to edges, edge contrast and landscape fragmentation: interactions affecting farmland birds around forest plantations. Biol Conserv 142:824–838
Ries L, Debinski DM (2001) Butterfly responses to habitat edges in the highly fragmented prairies of Central Iowa. J Anim Ecol 70:840–852
Ries L, Sisk TD (2010) What is an edge species? The implications of sensitivity to habitat edges. Oikos 119:1636–1642
Ries L, Fletcher RJJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Ries L, Murphy SM, Wimp GM, Fletcher RJ (2017) Closing persistent gaps in knowledge about edge ecology. Curr Landsc Ecol Rep 2:30–41
Root RB, Kareiva PM (1984) The search for resources by cabbage butterflies (Pieris rapae): ecological consequences and adaptive significance of markovian movements in a patchy environment. Ecology 65:147–165
Ruffell J, Didham RK (2016) Towards a better mechanistic understanding of edge effects. Landsc Ecol 31:2205–2213
Samways MJ (2007) Implementing ecological networks for conserving insect and other biodiversity. In: Stewart AJA, New TR, Lewis OT (eds) Insect Conservation Biology. Springer, Netherlands, pp 127–143
Samways MJ, Bazelet CS, Pryke JS (2010) Provision of ecosystem services by large scale corridors and ecological networks. Biodivers Conserv 19:2949–2962
Schtickzelle N, Joiris A, Van Dyck H, Baguette M (2007) Quantitative analysis of changes in movement behaviour within and outside habitat in a specialist butterfly. BMC Evol Biol 7:1–15
Schultz CB, Crone EE (2001) Edge-mediated dispersal behavior in a prairie butterfly. Ecology 82:1879–1892
Schultz CB, Franco AMA, Crone EE (2012) Response of butterflies to structural and resource boundaries. J Anim Ecol 81:724–734
Shreeve TG, Dennis RLH (2011) Landscape scale conservation: resources, behaviour, the matrix and opportunities. J Insect Conserv 15:179–188
Siu JC, Koscinski D, Keyghobadi N (2016) Swallowtail butterflies show positive edge responses predicted by resource use. Landsc Ecol 31:2115–2131
Tscharntke T, Tylianakis JM, Rand TA et al (2012) Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biol Rev 87:661–685
Turner JRG, Gatehouse CM, Corey CA (1987) Does solar energy control organic diversity? Butterflies, moths and the British climate. Oikos 48:195–205
van Halder I, Barbaro L, Jactel H (2011) Conserving butterflies in fragmented plantation forests: are edge and interior habitats equally important? J Insect Conserv 15:591–601
van Schalkwyk J, Pryke JS, Samways MJ (2017) Wide corridors with much environmental heterogeneity best conserve high dung beetle and ant diversity. Biodivers Conserv 26:1243–1256
van Schalkwyk J, Pryke JS, Samways MJ (2019) Contribution of common vs. rare species to species diversity patterns in conservation corridors. Ecol Indic 104:279–288
van Schalkwyk J, Pryke JS, Samways MJ, Gaigher R (2020a) Environmental filtering and spillover explain multi – species edge responses across agricultural boundaries in a biosphere reserve. Sci Rep 10:1–10
van Schalkwyk J, Pryke JS, Samways MJ, Gaigher R (2020b) Spillover of terrestrial arthropod species and beta diversity in perennial crops relative to spatial scale of land-use intensity. J Appl Ecol. https://doi.org/10.1111/1365-2664.13638
van Schalkwyk J, Pryke JS, Samways MJ, Gaigher R (2020c) Corridor width determines strength of edge influence on arthropods in conservation corridors. Landsc Ecol. https://doi.org/10.1007/s10980-020-01008-6
van Schalkwyk J, Gaigher R, Pryke JS, Samways MJ (2021a) Within-corridor heterogeneity is more important than corridor design for maintaining butterfly functional and taxonomic diversity. J Appl Ecol. https://doi.org/10.1111/1365-2664.14006
van Schalkwyk J, Pryke JS, Samways MJ, Gaigher R (2021b) Maintaining high vegetation structural diversity in the landscape promotes arthropod diversity in dynamic production areas. Landsc Ecol 36:1773–1785
Vlašánek P, Fric ZF, Zimmermann K, et al (2018) Do butterfly activity data from mark-recapture surveys reflect temporal patterns? J Insect Behav 31:385–401
Watling JI, Orrock JL (2010) Measuring edge contrast using biotic criteria helps define edge effects on the density of an invasive plant. Landsc Ecol 25:69–78
Wikstroem L, Milberg P, Bergman K-O (2009) Monitoring of butterflies in semi-natural grasslands: diurnal variation and weather effects. J Insect Conserv 13:203–211
Wittman J, Stivers E, Larsen K (2017) Butterfly surveys are impacted by time of day. J Lepid Soc 71:125–129
Wood PA, Samways MJ (1991) Landscape element pattern and continuity of butterfly flight paths in an ecologically landscaped botanic garden, Natal, South Africa. Biol Conserv 58:149–166
Woodhall S (2005) Field guide to butterflies of South Africa. Struik Nature, Cape Town
Yekwayo I, Pryke JS, Roets F, Samways MJ (2016) Surrounding vegetation matters for arthropods of small, natural patches of indigenous forest. Insect Conserv Divers 9:224–235
Acknowledgements
This research was funded by Mondi Group. The authors thank L. Davids for field assistance. Butterfly collecting was approved by Ezemvelo KwaZulu-Natal Wildlife under Permit Number OP480/2021.
Author information
Authors and Affiliations
Contributions
JS, JSP, MJS, and RG conceived and designed the study. JS collected the data, conducted the analysis, and was the primary author of the manuscript. All authors contributed critically to draft manuscripts and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Schalkwyk, J., Pryke, J.S., Samways, M.J. et al. Corridor width and orientation are complementary design variables for butterflies in conservation corridors. Landsc Ecol 37, 2535–2549 (2022). https://doi.org/10.1007/s10980-022-01484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01484-y