Abstract
Context
Although the spatiotemporal dynamics of spruce budworm outbreaks have been intensively studied, forecasting outbreaks remains challenging. During outbreaks, budworm-linked warblers (Tennessee, Cape May, and bay-breasted warbler) show a strong positive response to increases in spruce budworm, but little is known about the relative timing of these responses.
Objectives
We hypothesized that these warblers could be used as sentinels of future defoliation of budworm host trees. We examined the timing and magnitude of the relationships between defoliation by spruce budworm and changes in the probability of presence of warblers to determine whether they responded to budworm infestation before local defoliation being observed by standard detection methods.
Methods
We modelled this relationship using large-scale point count surveys of songbirds and maps of cumulative time-lagged defoliation over multiple spatial scales (2–30 km radius around sampling points) in Quebec, Canada.
Results
All three warbler species responded positively to defoliation at each spatial scale considered, but the timing of their response differed. Maximum probability of presence of Tennessee and Cape May warbler coincided with observations of local defoliation, or provided a one year warning, making them of little use to guide early interventions. In contrast, the probability of presence of bay-breasted warbler consistently increased 3–4 years before defoliation was detectable.
Conclusions
Early detection is a critical step in the management of spruce budworm outbreaks and rapid increases in the probability of presence of bay-breasted warbler could be used to identify future epicenters and target ground-based local sampling of spruce budworm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All animal populations fluctuate through space and time (Lundberg et al. 2000). However, in some species, these fluctuations entail dramatic oscillations in density over several orders of magnitude (Kendall 1998). Of particular interest in this regard are outbreaking forest insect pests in the order Lepidoptera, whose spatially synchronous population fluctuations affect large geographic areas (Cooke et al. 2007; Haynes et al. 2009; Kneeshaw et al. 2015). These species have been intensively studied because of the significant effects outbreaks have on forest landscape structure and dynamics (Liebhold et al. 2000; Speer et al. 2001; Cooke and Lorenzetti 2006; Myers and Cory 2013; Walter et al. 2016). Despite the fact that outbreaks affect forest ecosystem functioning (Maguire et al. 2015) and have severe economic impacts (Chang et al. 2012), we remain relatively ill-equipped to forecast where and when outbreaks will occur.
Insect outbreaks also represent a significant and spatially complex pulsed resource (Yang et al. 2008) in boreal forest systems and result in significant changes in community assemblage (Eveleigh et al. 2007; Smith et al. 2011; Marrec et al. 2018). While such community-level responses have been documented during and following outbreaks in multiple systems (Morris et al. 1958; Carson and Root 2000; Holmes et al. 2009), we know surprisingly little about the relative timing of these effects. A better understanding of the temporal consequences of insect outbreaks is essential to interpret and predict landscape-scale biodiversity responses to outbreaks, that can be used to develop reliable forecasting methods to guide management actions.
As outbreaks of forest insect pests tend to spread very quickly and occur over very large spatial scales, management strategies have typically aimed to limit damage (i.e., foliage protection) rather than eradicate the pest (Régnière et al. 2001). More recently, Early Intervention Strategies (EIS) have been proposed to control populations at low densities using pesticides such as Btk and Mimic (MacLean et al. 2019). This approach is based on the idea that outbreaks are more likely to be contained before they exceed a certain threshold population density (Raffa et al. 2008; Johns et al. 2019), although identifying this threshold is not trivial. Effective early intervention requires accurate methods to detect where and when low-density, near threshold populations occur. However, identifying such incipient outbreaking populations remains challenging in widespread forest insects that exhibit significant delays between critical population increases and observable defoliation.
The eastern spruce budworm [Choristoneura fumiferana (Clem.)] is an outbreaking native defoliator of spruce (Picea spp.) and fir (Abies balsamea) trees in North America that causes significant forest mortality (Candau et al. 1998; Sturtevant et al. 2015). In eastern Canada, spruce budworm populations erupt synchronously over very large spatial scales (i.e. more than 13 million ha affected by the current outbreak) at intervals of ~ 35–40 years (Royama 1984; Boulanger and Arseneault 2004). The species is univoltine. Adults lay eggs at the end of summer that hatch and overwinter as stage 2 larvae. The larvae emerge from hibernacula in the following spring to feed on the new foliage of host trees. Balsam fir is the primary host species, but black spruce is also used as a secondary host (Hennigar et al. 2008). Over a period of approximately six weeks larvae complete a total of five more larval stages before molting to a pupa. The 5th and 6th instar larva consume more foliage than the other four stages. Moths emerge, mate and lay eggs on the host trees in late summer. Importantly, the population is building-up over several generations (i.e. several years) and damages on host foliage are visible with a significant delay, which makes early intervention strategies crucial for this system. Further details on the spruce budworm life cycle can be found in Régnière and Nealis (2007).
In the boreal forests of eastern Canada, early intervention are crucial and holds great promise to reduce the negative consequences of spruce budworm outbreaks (MacLean et al. 2019). However, early detection of incipient outbreak “hotspots” has proven difficult or impractical with current monitoring techniques. Spruce budworm populations are currently monitored using ground-based surveys of second-instar larvae (L2s) conducted in the fall (MacLean et al. 2019), and although these surveys can detect small, local changes in abundance, they are costly, time-consuming, and difficult to apply in low density populations over large areas. In contrast, remote sensing approaches can cover large areas but are unable to detect low population densities, making them of limited use for guiding early intervention (MacLean and MacKinnon 1996; Rahimzadeh-Bajgiran et al. 2018).
Changes in insect population densities have the potential to be used to identify areas of rising outbreaks before they become detectable by other methods. The use of animal sentinels for environmental monitoring is an increasing area of research, especially in the context of tracking the influence of climate change and global disturbances (Wikelski and Tertitski 2016). Spruce budworm outbreaks are known to elicit a numerical response in the population densities of several insectivorous bird species by providing a pulse in food availability (Yang et al. 2008; Venier and Holmes 2010). Specifically, three warbler species (Tennessee warbler, Leiothlypis peregrina; Cape May warbler, Setophaga tigrina; and bay-breasted warbler, S. castanea) have shown particularly strong and consistent responses, with 4–12 fold increases in density over 4–5 years following the onset of an outbreak (Kendeigh 1947; Morse 1978; Holmes et al. 2009; Venier et al. 2009; Venier and Holmes 2010). The effects of the budworm subsidy on bird populations can be dramatic. One study reported that although these warblers were absent during non-outbreak years, they represented 25% of the bird population during the outbreak peak (Holmes et al. 2009). These species are known to switch to late-instar spruce budworm larvae as a food source during outbreaks (Venier and Holmes 2010). For this reason, these three species are referred to as budworm-linked warblers (for a review, see Venier and Holmes 2010).
Most studies examining the response of budworm-linked warblers to increasing spruce budworm densities have been conducted at a local, stand scale after the outbreak has peaked (Morris et al. 1958; Sanders 1970; Morse 1978). More recently, Drever et al. (2018) investigated larger scale relationships between spruce budworm defoliation and the abundance of eight warbler species, including budworm-linked warblers. Using data from the North American Breeding Bird Survey, they found that birds tended to increase in population density in response to budworm outbreaks at the scale of individual survey routes (ca. 40 km-long). At that spatial scale, warbler densities responded most strongly 1 year prior to spruce budworm defoliation became visible. The presence or abundance of budworm-linked warblers may therefore hold great potential as an early indicator of future spruce budworm defoliation. However, we do not know the temporal lag between outbreak onset and bird numerical response, nor do we know the spatial scale at which bird populations respond to developing outbreaks.
In this study, we investigated whether budworm-linked warblers could be use as sentinels of future defoliation. We characterized the timing and magnitude of change in the probability of presence of each species of budworm-linked warbler prior to defoliation caused by spruce budworm outbreaks at multiple spatial and temporal scales. Specifically, we modelled the relationship between time-lagged, cumulative spruce budworm defoliation and the probability of presence of three bird species known for their strong numerical response to budworm outbreaks: Tennessee warbler, Cape May warbler, and bay-breasted warbler. By comparing the relative timing of response of these three bird species to insect defoliation, we sought to determine which, if any, could be used as effective early indicators of future defoliation. It is important to note that although warbler densities do increase in response to outbreaks, they have little to no influence on budworm outbreak dynamics (Crawford and Jennings 1989; Venier and Holmes 2010). Here, we do not consider budworm-linked warblers as a potential control tool, but instead examine their utility as sentinels to identify areas of increasing budworm populations to guide early intervention strategies for budworm control.
We predict that budworm-linked warblers will show a positive response to budworm population increases before defoliation is visible from standard aerial surveys, and that these increases will vary among species. MacArthur (1958) showed that Cape May warbler nests and forages primarily near the tree top, where spruce budworm larvae begin feeding (Baskerville 1975), thus leading to the prediction that Cape May warbler will respond to a developing outbreaks earlier than other species. Alternatively, Holmes et al. (2009) observed that bay-breasted warbler increased earlier than the other two budworm-linked warblers during the previous outbreak at a site in northern Ontario, suggesting that this species would increase first in abundance as a result of a better exploitation of budworm larvae at low densities. By comparing these species and identifying the spatial and temporal scales at which they respond to developing outbreaks, we aimed to investigate whether these budworm-linked warblers could be used as sentinels to inform management of this landscape-scale, economically important phenomenon.
Methods
Study area
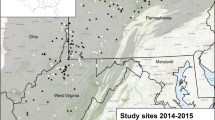
The current outbreak was first detected in 2006 on the North Shore of the St. Lawrence River in Québec, Canada. By 2017, this outbreak had expanded to > 13 million ha (Ministère des Forêts 2020). We sought to leverage existing bird surveys conducted over multiple years within this disturbed landscape (Fig. 1) to investigate spatiotemporal associations between the spruce budworm and budworm-linked warblers. The study area is located in the eastern boreal forest (Rowe 1972) and covers approximately 161,740 km2. Black spruce (Picea mariana) and balsam fir (Abies balsamea) are typically codominant in the forest canopy. In the North Shore region, two bioclimatic subdomains characterize the continuous boreal forest: balsam fir–white birch near the St-Lawrence, and black spruce–feather moss north of 49° (Thibault and Hotte 1985; Robitaille and Saucier 1998). Balsam fir dominates on mesic sites in the south, in association with white birch and trembling aspen, whereas black spruce abundance increases with latitude. The topography is generally rugged, with high hills and steep valleys with rivers flowing from northwest to southeast. The dominant surficial deposits are tills, with thicker deposits in depressions and on lower slopes and thin deposits on slopes and some summits (Robitaille and Saucier 1998).
Bird surveys and Kernel density estimation
Forest birds were surveyed between 2006 and 2012 using the point count method (Reynolds et al. 1980; Blondel et al. 1981). Bird surveys were conducted in the study area, originally as part of impact assessments studies of several projects (Fig. 1). Each point was surveyed in one year as the area was difficult to access and most sampling site was only accessible by helicopter. Within-year point count surveys were separated by at least 250 m to reduce the likelihood of double-counting. All birds detected by sight or sound were recorded during a 15 min period. The distance to each detected bird was estimated as one of three categories: < 75 m, 75 to 100 m, or > 100 m. Only birds detected within a 75 m radius were included in the analysis to reduce the influence of differences in detectability among the three species. The detection radii of the three species have previously been estimated to be 65 m for Tennessee warbler, 42 m for Cape May warbler and 43 m for bay-breasted warbler (Matsuoka et al. 2012). Counts were performed during the peak of the breeding season, between early June and mid-July, between dawn and 10:40 EDT.
All counts were conducted under favorable weather conditions (i.e., low wind and no steady rain) and all observers were experienced at bird identification by sight and sound as well as distance estimation. Basic vegetation data were also collected by observers at the time of bird surveys. Only point counts conducted where vegetation was deemed suitable (i.e. that the forest type contained host species of the spruce budworm; spruce woodland, mixed woodland, balsam fir) for the focal species (N = 585 points) were retained for analyses. We excluded counts performed in burns, peatlands, low productivity black spruce-lichen stands, and on rocky outcrops. Sites located beyond the northern tree limit were also excluded from the analysis.
We modeled the relationship between warbler probability of presence and spatiotemporal context, including years before defoliation was visible, using logistic regression. We chose to use the probability of presence because (i) abundance are more likely to be underestimated and are more observer-dependent, (ii) change in the probability of presence is expected to be the first indicator to capture bird response to spruce budworm outbreak. To reduce the potential bias associated with many absences (i.e., zero-inflation) and to limit the proportion of point counts falling outside the main distribution of these species (i.e., false absences), the set of bird presences and absences was restricted to an expected geographic distribution of each species. Expected distributions were estimated using a kernel density function (Worton 1989) with which we transformed a discontinuous point distribution layer (i.e., bird presence) into a continuous density surface (raster) using a weighted, variable-radius two-dimensional kernel. Using the 77 point count stations where Cape May warbler was detected and the 111 where bay-breasted warbler was detected (Fig. 2), we calculated raster surfaces representing the intensity of probability of presence of Cape May warbler and bay-breasted warbler. We used the kernelUD function of the adehabitatHR package (Calenge 2006) in R Core Team (2017) and an ad hoc technique for the calculation of the smoothing factor h (Millspaugh and Marzluff 2001). Raster resolution was set at 250 m. The resulting isopleth values describe the probability of encountering each species within each raster cell (i.e., “location”). Species distributions consisted of all the sampling points in the 100% density isopleth. This resulted in the selection of 441 locations for Cape May warbler, including the 77 point count stations where this species was confirmed present, and 364 locations where it was not. Similarly, this process identified 405 locations for bay-breasted warbler, including the 111 point count stations where bay-breasted warbler was confirmed present and 294 locations where it was not. For Tennessee warbler, as all the locations (N = 585 points) were included within the 100% isopleth, we used the entire study area (i.e. N = 201 sampling points where this species was detected and N = 384 locations where it was not; Fig. 2).
Spatial scales and spruce budworm defoliation
We investigated the temporal response of budworm linked warblers to defoliation at multiple time lags and multiple spatial scales. Digital vector format aerial defoliation survey data (Ministère des Forêts 2020) were used as a proxy for moderate to high spruce budworm populations. Areas classified as defoliated in a given year were assumed to have hosted spruce budworm at densities insufficient to cause visible defoliation in preceding years. We calculated cumulative spruce budworm defoliation (percent area of the buffer affected) around each point count station at four spatial scales (2, 5, 10, 30 km radius buffers), for five different defoliation time lags: from the year of bird count (t) to four years after bird count (t + 4). Buffers that overlapped with the St. Lawrence River were clipped and cumulative defoliation was calculated as the percentage of the clipped area defoliated using ARCGIS 10.5 (ESRI, Redlands, California, USA). For example, to calculate cumulative defoliation two years after a bird count conducted in 2010 (i.e. t + 2 defoliation time lag), we summed all areas that had been classified as defoliated by 2012 and divided this value by the buffer area considered.
Bird habitat
The presence of suitable habitat is also likely to influence the presence of insectivorous warblers. To distinguish the influence of habitat from that of spruce budworm defoliation, we included bird habitat attributes as predictors in our models of bird response to time-lagged defoliation. Each sampling location was characterized using three habitat variables deemed important for all focal species: stand height, canopy closure, and forest stand type (Baltz and Latta 2020; Venier et al. 2011; Rimmer and McFarland 2012). Data were derived from provincial forest inventory GIS layers (Ministère des Forêts 2020). Stand height classes of the forest inventory were grouped into three categories (< 7 m, 7–12 m, or > 12 m). Canopy closure was described by four classes: 25–40%, 40–60%, 60–80%, or > 80%. Finally, forest stand type was classified as coniferous, deciduous, or mixed, according to the percentage of coniferous trees. Coniferous stands had more than 75% of their basal area in coniferous species, whereas in deciduous stands, this proportion was between 0 and 25% (Berger and Leboeuf 2015).
Model building and comparison
Our goal was to characterize the early temporal response of Tennessee warbler, Cape May warbler, and bay-breasted warbler to spruce budworm defoliation at different spatial scales, using five time lags between bird count and defoliation at each point count station. Each model included habitat associated with the probability of presence of each bird species. We considered each spatial scale separately and performed distinct analyses at each spatial scale. Models were built in two steps. We first built habitat-only models to identify habitat variables relevant to each species. The binary presence-absence of each species was used as the response variable in a generalized linear mixed model framework using a binomial error distribution (i.e., logistic regression). To control for possible temporal variation in factors affecting the presence of each species, we included year as a random factor. We selected the best habitat model from among the set of possible habitat models using the Akaike information Criterion (AIC) (Burnham and Anderson 2002). When several models performed equally well (i.e., ∆AIC < 2), we selected the model with the fewest retained predictors.
In the second step, we investigated whether our habitat-only models could be improved by the addition of time-lagged cumulative historical spruce budworm defoliation. We built a series of five models—one for each defoliation time lag considered. The different time lags were not included in the same model because collinearity in these nested predictors have the potential to confound interpretation and the signs of individual coefficients. Further, we sought to identify the single relative best time lag that captured the response of each warbler species to budworm populations by identifying the model(s) that was best supported by our data. We compared each time-lagged defoliation model for each species to the associated (i.e., species-specific) habitat-only null model using AIC. Lagged defoliation models with a ∆AIC > 10 (Burnham and Anderson 2002) relative to the null model were considered as evidence that defoliation at a given time lag improved the prediction of warbler probability of presence. Models with the lowest AIC values were considered best supported by the data. Models and, therefore, defoliation time lags within a ∆AIC of two were considered to perform equally well (Burnham and Anderson 2002). Prior to performing the analysis, all time lag variables were standardized to zero mean and unit variance. Both steps were repeated separately for each species and each spatial scale but only results for the 2 km spatial scale are shown. However, for each time lag, the proportion of the buffer defoliated among the different spatial scales was highly correlated (i.e. ≥ 0.6) and, therefore, we only show the results at the 2 km spatial scale as results were similar at all spatial scales (Supporting information Fig. S1 in Appendix S1). Results at 5, 10 and 30 km radii are provided in Supporting information in Appendix S2.
Model performance
We assessed model performance using the receiver operating characteristic (ROC) analysis and the area under the ROC curve (AUC) statistic (Fielding and Bell 1997). To generate ROC curves, data were randomly divided into training (70%) and testing (30/%) datasets. Random splitting of the data into training and testing sets was repeated 1000 times to generate confidence intervals around our ROC curve estimates. For more details about the procedure, see Appendix S4. All statistical analysis were performed using R Statistical Software (R Core Team 2017).
Results
Tennessee warbler
Stand height and canopy closure were retained in the best habitat model of Tennessee warbler probability of presence (Appendix S1: Table S1a). The probability of presence of Tennessee warbler increased with stand density (i.e., with a canopy closure of at least 60%) and decreased with stand height (Appendix S3: Fig. S1a, b). Lagged cumulative spruce budworm defoliation improved the “habitat-only” model of Tennessee warbler presence (i.e. ∆AIC > 10; Fig. 3a): cumulative defoliation one year after bird counts and the year of bird counts had the greatest influence on Tennessee warbler probability of presence (Table 1a). Hence, the probability of presence of Tennessee warblers increased with spruce budworm abundance a year before defoliation was visible, or the same year.
Cape May warbler
The probability of presence of Cape May warbler increased with canopy closure (Appendix S3: Fig. S2). No other habitat variable was retained (Appendix S3: Table S1b). Lagged cumulative spruce budworm defoliation improved the “habitat-only” model of Cape May warbler presence (i.e. ∆AIC > 10; Fig. 3b): cumulative defoliation one year after bird counts and in the year of bird counts had the greatest influence on Cape May warbler probability of presence (Table 1b).
Bay-breasted warbler
The probability of presence increased with stand density (Appendix S3: Fig. S3a) and was highest in mixed conifer-deciduous stands and lowest in deciduous stands (Appendix S3: Fig. S3b). As with Tennessee warbler and Cape May warbler, lagged defoliation improved the habitat model for the bay-breasted warbler (Fig. 3c), but the best model suggested an earlier response to increasing spruce budworm defoliation. Models with cumulative defoliation observed 3–4 years after bird counts performed equally well (Table 1c). In other words, bay-breasted warbler showed an increasing presence to increasing spruce budworm populations 3–4 years before defoliation was visible at the point count station.
Fitted probabilities of presence
To visualize the expected response of budworm-linked warblers to spruce budworm defoliation at the time lag associated with the best model, we calculated fitted probabilities of presence for each species. Both Tennessee warbler and Cape May warbler showed a similar pattern of increase in their fitted probability of presence as a function of the cumulative area defoliated in a 2 km buffer. This probability was highest when defoliation reached 80–100% one year after the bird count (Fig. 4a, b). For bay-breasted warbler, the fitted probability of presence was lower than for Tennessee warbler and Cape May warbler, but it peaked more markedly with spruce budworm defoliation, showing a tenfold increase when cumulative defoliation increased from 0 to 20 to more than 80%, 4 years after the count (Fig. 4c).
Model performance
All models performed reasonably well. The AUC statistic and its standard deviation over 1000 replicates was 0.76 ± 0.04 for the Tennessee warbler model, and 0.84 ± 0.04 for both Cape May warbler and bay-breasted warbler models (Appendix S5: Fig. S1a, b, c).
Discussion
Our findings indicate that birds hold great potential as early indicators of developing outbreaks, which will help guide forest managers to respond to or pre-empt large-scale forest insect disturbances. All three species of budworm-linked warblers responded positively to defoliation, used as a proxy of budworm populations, before it became visible from aerial surveys. That is, as hypothesized, we found that bird population dynamics tracked those of the spruce budworm and could help us identify where and when outbreaks are developing and more efficiently guide ground based L2 survey, before those outbreaks are detectable using aerial budworm monitoring methods. It could be argued that birds actually responded to landscape features correlated with spruce budworm that were not included in our habitat-only model. For example, stands at the epicentres of the northeast Canadian outbreak have been shown to share some common characteristics, such as a high concentration of balsam fir (see Bouchard and Auger 2014). However, if those landscape features were a strong driver of bird response, we would not detect any temporal difference linked to spruce budworm defoliation since those landscape features, such as the proportion of balsam fir, are consistent over the temporal scale considered here (i.e. they change only at a decadal scale).
Budworm-linked warblers also responded to defoliation at multiple spatial scales. In other words, they responded to increases in spruce budworm at both local and broader spatial scales. However, the precise timing and strength of this response to population increases of spruce budworm was species-specific. The presence of bay-breasted warbler provided a reliable indication of future defoliation by spruce budworm 3–4 years in advance. This species thus tracked low population levels of spruce budworm before the insect had caused defoliation detectable from the ground or from the air. Its probability of presence increased more than tenfold as many as 4 years prior to a major shift in cumulative defoliation within a 2-km radius of a sampling point. In contrast, the responses of the two other budworm-linked warblers coincided with local defoliation or provided only a one-year warning, making them of little use to guide early interventions.
Although we focused on the probability of presence of each warbler species rather than on their abundance and were, therefore, unable to directly quantify the increase in birds number, our results are consistent with previous studies reporting a positive abundance response of each budworm-linked warbler species to spruce budworm defoliation (see Venier and Holmes 2010 for a review). We build upon and extend these studies by exploring bird response to defoliation at several temporal and spatial scales simultaneously. The fact that presence of bay-breasted warbler was linked to the proportion of budworm defoliation within a 2 km radius indicates that bay-breasted warbler response is a landscape-level phenomenon. Our test of multiple scales of response was because we did not know at what scale birds would respond and thus, our findings show that birds respond at multiple scales probably as the spruce budworm populations are also increasing across multiple scales. This finding could be especially helpful to managers to orient ground-based larval sampling and for the development of new early intervention strategies. For example, relative to field based L2 surveys, automated acoustic recorders are an effective, low-cost approach to monitor bird species over large and temporal scales (Thomas and Marques 2012; Wimmer et al. 2013). This approach could be particularly interesting in the context of early intervention strategies, especially in remote areas such as our study area, to monitor bird communities and detect changes in the presence of budworm-linked warblers.
Our results also highlight the importance of the temporal component of songbird responses to spruce budworm outbreaks. Differences among species in their timing of response to incipient outbreaks have only been described in a single site (MacArthur 1958; Morse 1978; Holmes et al. 2009; Venier et al. 2009; Venier and Holmes 2010). To our knowledge, this is the first study investigating the timing of response of budworm-linked warblers to a developing outbreak at different spatial scales. Spruce budworm defoliation typically begins at the top of the tree crown and spreads downwards (Baskerville 1975; Virgin et al. 2018). Because Cape May warbler forages consistently near the top of trees, in upper and outer branches of spruce and fir (MacArthur 1958; Baltz and Latta 2020), we expected that species to be the earliest to respond. However, bay-breasted warbler actually responded earlier than either Tennessee warbler or Cape May warbler. At a local scale (10.4 ha study plot), Holmes et al. (2009) also reported that bay-breasted warbler was the first species to show an increase in abundance following the onset of a spruce budworm outbreak, even though defoliation was still low (< 10%) in the plot and in the surrounding region. At the southern edge of the current outbreak, Perrier et al. (2021) also found that bay-breasted warbler’s abundance increased earlier than those of Cape May warbler and Tennessee warbler. At a regional scale, Drever et al. (2018) also reported that bay-breasted warbler counts increased by 260% when defoliation had taken place over the 4 previous years. In contrast, Tennessee warbler and Cape May warbler showed similar local responses and only increased shortly before local defoliation. Hence, the presence of bay-breasted warbler appears to be a reliable indicator of future local defoliation by spruce budworm.
Differences in the temporal responses of the three budworm-linked warbler species may reflect contrasting demographic responses. It has been suggested that bay-breasted warbler’s foraging niche may allow this species to exploit budworm at lower densities, because it uses a broader range of heights than Cape May warbler (MacArthur 1958). When foraging, bay-breasted warbler also tends to move more slowly, spending more time on each branch than either Cape May warbler or Tennessee warbler. This may reflect the fact that it mainly forages on the inner section of trees rather than at the tip of branches, allowing a more thorough search when preys are at low density (MacArthur 1958; Venier et al. 2011). Interspecific competition is unlikely to affect these dynamics as each species has its own foraging niche (MacArthur 1958; Venier et al. 2009; Venier and Holmes 2010) and that during an outbreak food is not a limiting resource.
Increases in warbler presence in response to budworm defoliation could reflect three non-mutually exclusive mechanisms. First, increased reproductive performance, through an increase in both clutch size and an improvement in the body condition of fledglings, potentially increasing recruitment (MacArthur 1958). In birds, as in many other taxa, one of the main factors determining reproductive success, after nest predation, is food abundance (e.g. Holmes et al. 1986; Dias and Blondel 1996; Verboven et al. 2001; Durant et al. 2003). Insect outbreaks represent an enormous resource supplement and the numerical response of predators to such outbreaks is well documented (e.g. Eveleigh et al. 2007; Bouchard et al. 2008). Spruce budworm does not become attractive to most species of birds until the L4 instar and most of the predation actually occurs on L5 and L6 instars (Morris et al. 1958). It has been estimated that large larvae and pupae were available to birds between the middle of June and the end of July (Crawford and Jennings 1989). This coincides with the breeding season and, specifically, with the period during which budworm-linked warblers feed their nestlings or fledglings in these northern regions (Baltz and Latta 2020; Venier et al. 2011; Rimmer and Mcfarland 2012). Then, it is reasonable to expect that this pulse in food supply will result in an increased reproductive success.
Second, dispersal could explain the observed increase in the probability of presence of budworm-linked warblers in the years preceding visible defoliation. Dispersal have, for example, been implicated in the numerical responses of woodpeckers following outbreaks of the mountain pine beetle (Dendroctonus ponderosae) in British Columbia (Edworthy et al. 2011). Perrier et al. (2021) found that first-time breeding bay-breasted warblers were associated with lower densities of spruce budworm larvae than older males, suggesting that sites at early outbreak stages are detected through natal dispersal. Long-distance dispersal of budworm-linked warblers may facilitate tracking the rapid expansion of spruce budworm outbreaks and the appearance of new, spatially discontinuous hotspots through, notably the sharing of social information (Valone and Templeton 2002). Songbirds have been shown to use location cues (Betts et al. 2008; Thériault et al. 2012) or the reproductive success of conspecifics (i.e. public information, e.g. Doligez et al. 2002; Arlt and Pärt 2008; Betts et al. 2008; Boulinier et al. 2008) to base their settlement decisions. This type of information may be particularly useful for inexperienced individuals that cannot rely on their own experience, but data from eastern Québec suggest that adult budworm-linked warblers showed low site fidelity from year to year, possibly tracking high larvae density (Moisan Perrier et al. 2021). Public information use has been suggested elsewhere to explain tracking of spruce budworm through dispersal in Tennessee and Cape May warblers (MacArthur 1958; Royama 1984; Holmes et al. 2009; Venier et al. 2009).
A third mechanism that may help explain early detection of new or developing spruce budworm outbreaks by budworm-linked warblers is that of chemical cues produced by trees being fed upon. In the autumnal moth (Epirrita autumnata) outbreak system, volatile organic compounds released by host trees upon their attack attract insectivorous songbirds (Mäntylä et al. 2008). However, the numerical response of locally-breeding songbirds and the distance over which these volatile compounds can be detected remains to be determined. The same type of host-tree volatile induced aggregation dynamic could cause warblers to be attracted to areas undergoing spruce budworm defoliation. This mechanism is very similar to the so-called “bird feeder effect” hypothesis of Eveleigh et al. (2007) that describes the spatial aggregation of parasitoid natural enemies in response to developing outbreak epicenters.
Conclusions
Spruce budworm outbreak duration and severity are expected to increase in eastern Canada in the near future (Gray et al. 2000). In response to climate change, the geographic range of the insect is also expected to shift such that outbreaks may occur in regions where they were not observed in the past (Pureswaran 2015). Early detection is a critical step in the management of spruce budworm outbreaks in such a changing environment. Spatial and temporal patterns in the presence of budworm-linked warblers may represent a complementary tool to identify future outbreak epicenters and to guide ground surveys and management actions. Our study is the first to investigate how budworm-linked warblers respond to spruce budworm outbreaks and associated defoliation at multiple temporal and spatial scales. We found that bay-breasted warbler can be used as an early warning sentinel of rising spruce budworm populations up to four years before defoliation becomes visible from aerial surveys. Additional work is still required to determine how to best operationalize this information into large-scale budworm population monitoring. Nonetheless, we envisage a system where targeted bird surveys conducted with the help of automated recording units are integrated into existing early instar larval sampling and pheromone-based adult moth trapping. Sectors characterized by high probabilities of presence of bay-breasted warbler (combined with high counts of L2 larvae) could be targeted for early intervention using biological insecticides.
Our findings provide several possible directions for future work regarding the mechanisms underlying avian response to increasing spruce budworm populations. It will first be important to determine whether surveys based on automated recording units provide the same 3–4 year warning signal by bay-breasted warbler as did our point count surveys. Other outstanding questions include: do birds increase their reproductive output locally or do large-scale dispersal movements allow individuals to track the advance of the outbreak? If both processes are involved, what is the relative importance of each one and does this relationship vary spatially and temporally? Finally, the threshold spruce budworm density at which warblers show a high increase in their probability of presence needs to be determined. It will be particularly interesting to determine whether this density differs from the current threshold density of four L4 larvae per 45 cm branch tip (MacLean et al. 2019) currently used to trigger early intervention population control.
References
Arlt D, Pärt T (2008) Post-breeding information gathering and breeding territory shifts in Northern wheatears. J Anim Ecol 77:211–219
Baltz ME, Latta SC (2020) Cape May warbler (Setophaga tigrina). In: The birds of the world (P.G. Rodewald, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA
Baskerville G (1975) Spruce budworm: the answer is forest management: or is it? For Chron 51:157–160
Berger J-P, Leboeuf A (2015) Norme de stratification écoforestière: quatrième inventaire écoforestier du Québec méridional. Ministère des forêts, de la faune et des parcs, Secteur des forêts, Direction des inventaires forestiers
Betts MG, Hadley AS, Rodenhouse N, Nocera JJ (2008) Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc R Soc Lond B 275:2257–2263
Blondel J, Ferry C, Frochot B (1981) Point counts with unlimited distance. Stud Avian Biol 6:414–420
Bouchard M, Auger I (2014) Influence of environmental factors and spatio-temporal covariates during the initial development of a spruce budworm outbreak. Landscape Ecol 29:111–126
Bouchard M, Kneeshaw D, Bergeron Y (2008) Ecosystem management based on large-scale disturbance pulses: a case study from sub-boreal forests of western Quebec (Canada). For Ecol Manage 256:1734–1742
Boulanger Y, Arseneault D (2004) Spruce budworm outbreaks in Eastern Quebec over the last 450 years. Can J for Res 34:1035–1043
Boulinier T, McCoy KD, Yoccoz NG, Gasparini J, Tveraa T (2008) Public information affects breeding dispersal in a colonial bird: kittiwakes cue on neighbors. Biol Let 4:538–540
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach, 2nd edn. Springer, Verlag
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Candau JN, Fleming RA, Hopkin A (1998) Spatiotemporal patterns of large-scale defoliation caused by the spruce budworm in Ontario since 1941. Can J For Res-Revue Canadienne De Recherche Forestiere 28:1733–1741
Carson WP, Root RB (2000) Herbivory and plant species coexistence: community regulation by an outbreaking phytophagous insect. Ecol Monogr 70:73–99
Chang W-Y, Lantz VA, Hennigar CR, MacLean DA (2012) Economic impacts of forest pests: a case study of spruce budworm outbreaks and control in New Brunswick, Canada. Can J for Res 42:490–505
Cooke BJ, Lorenzetti F (2006) The dynamics of forest tent caterpillar outbreaks in Quebec, Canada. For Ecol Manage 226:110–121
Cooke BJ, Nealis VG, Régnière J (2007) Insect defoliators as periodic disturbances in northern forest ecosystems. In: Johnson EA, Miyanishi K (eds) Plant disturbance ecology: the process and the response. Elsevier Academic Press, Burlington, pp 487–525
Crawford HS, Jennings DT (1989) Predation by birds on spruce budworm Choristoneura fumiferana: functional, numerical, and total responses. Ecology 70:152–163
Dias PC, Blondel J (1996) Breeding time, food supply and fitness components of blue tits Parus caeruleus in Mediterranean habitats. Ibis 138:644–649
Doligez B, Danchin E, Clobert J (2002) Public information and breeding habitat selection in a wild bird population. Science 297:1168–1170
Drever MC, Smith AC, Venier LA, Sleep DJ, MacLean DA (2018) Cross-scale effects of spruce budworm outbreaks on boreal warblers in Eastern Canada. Ecol Evol 8:7334–7345
Durant JM, Anker-Nilssen T, Stenseth NC (2003) Trophic interactions under climate fluctuations: the Atlantic puffin as an example. Proc R Soc Lond B 270:1461–1466
Edworthy AB, Drever MC, Martin K (2011) Woodpeckers increase in abundance but maintain fecundity in response to an outbreak of mountain pine bark beetles. For Ecol Manage 261:203–210
Eveleigh ES et al (2007) Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc Natl Acad Sci USA 104:16976–16981
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24(1):38–49
Gray DR, Régnière J, Boulet B (2000) Analysis and use of historical patterns of spruce budworm defoliation to forecast outbreak patterns in Quebec. For Ecol Manage 127:217–231
Haynes KJ, Liebhold AM, Fearer TM, Wang G, Norman GW, Johnson DM (2009) Spatial synchrony propagates through a forest food web via consumer–resource interactions. Ecology 90:2974–2983
Hennigar CR, MacLean DA, Quiring DT, Kershaw JA Jr (2008) Differences in spruce budworm defoliation among balsam fir and white, red, and black spruce. For Sci 54:158–166
Holmes RT, Sherry TW, Sturges FW (1986) Bird community dynamics in a temperate deciduous forest: long-term trends at Hubbard Brook. Ecol Monogr 56:201–220
Holmes S, Sanders C, Fillman D, Welsh D (2009) Changes in a forest bird community during an outbreak cycle of the spruce budworm in Northwestern Ontario. Bird Populations 9:13–28
Johns RC et al (2019) A conceptual framework for the spruce budworm early intervention strategy: can outbreaks be stopped? Forests 10:910
Kendall B (1998) The macroecology of population dynamics: taxonomic and biogeographic patterns of population cycles. Ecol Lett 1:160–164
Kendeigh SC (1947) Bird population studies in the coniferous forest biome during a spruce budworm outbreak, vol 1. Department of Lands and Forests, Ontario
Kneeshaw D, Sturtevant BR, Cooke B, Work T, Pureswaran D, DeGrandpre L, MacLean DA (2015) Insect disturbances in forest ecosystems. In: Peh KS-H, Corlett RT, Bergeron Y (eds) Routledge handbook of forest ecology. Routledge, Oxfordshire
Liebhold A, Elkinton J, Williams D, Muzika R-M (2000) What causes outbreaks of the gypsy moth in North America? Popul Ecol 42:257–266
Lundberg P, Ranta E, Ripa J, Kaitala V (2000) Population variability in space and time. Trends Ecol Evol 15:460–464
MacArthur RH (1958) Population ecology of some warblers of Northeastern coniferous forests. Ecology 39:599–619
MacLean DA, MacKinnon WE (1996) Accuracy of aerial sketch-mapping estimates of spruce budworm defoliation in New Brunswick. Can J Res 26:2099–2108
MacLean DA, Amirault P, Amos-Binks L, Carleton D, Hennigar C, Johns R, Régnière J (2019) Positive results of an early intervention strategy to suppress a spruce budworm outbreak after five years of trials. Forests 10:448
Maguire DY, James PM, Buddle CM, Bennett EM (2015) Landscape connectivity and insect herbivory: a framework for understanding tradeoffs among ecosystem services. Glob Ecol Conserv 4:73–84
Mäntylä E, Alessio GA, Blande JD, Heijari J, Holopainen JK, Laaksonen T, Piirtola P, Klemola T (2008) From plants to birds: higher avian predation rates in trees responding to insect herbivory. PLoS ONE 3:e2832
Marrec R, Pontbriand-Paré O, Legault S, James PM (2018) Spatiotemporal variation in drivers of parasitoid metacommunity structure in continuous forest landscapes. Ecosphere 9:e02075
Matsuoka SM, Bayne EM, Sólymos P, Fontaine PC, Cumming SG, Schmiegelow FK, Song SJ (2012) Using binomial distance-sampling models to estimate the effective detection radius of point-count surveys across boreal Canada. Auk 129:268–282
Millspaugh JJ, Marzluff JM (2001) Radio-tracking and animal populations: past trends and future needs. Radio tracking and animal populations. Academic Press, San Diego
Ministère des Forêts (2020) Cartographie du 5e inventaire écoforestier du Québec méridional—Méthodes et données associées. Direction des inventaires forestiers, Québec
Moisan PJ, Kneeshaw D, St-Laurent MH, Pyle P, Villard M-A (2021) Budworm-linked warblers as early indicators of defoliation by spruce budworm: a field study. Ecol Indic 125:107543
Morris RF, Cheshire W, Miller CA, Mott DG (1958) The numerical response of avian and mammalian predators during a gradation of the spruce budworm. Ecology 39:487–494
Morse DH (1978) Populations of bay-breasted and Cape May warblers during an outbreak of the spruce budworm. Wilson Bulletin 90:404–413
Myers JH, Cory JS (2013) Population cycles in forest Lepidoptera revisited. Annu Rev Ecol Evol Syst 44:565–592
Perrier M, Kneeshaw D, St-Laurent MH, Villard M-A (2021) Site-infidelity by budworm linked warblers at the edge of an area defoliated by spruce budworm. Avian Conserv Ecol 16(1):17
Pureswaran DS et al (2015) Climate-induced changes in host tree–insect phenology may drive ecological state-shift in boreal forests. Ecology 96:1480–1491
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517
Rahimzadeh-Bajgiran P, Weiskittel AR, Kneeshaw D, MacLean DA (2018) Detection of annual spruce budworm defoliation and severity classification using Landsat imagery. Forests 9:357
Régnière J et al. Understanding of spruce budworm population dynamics: development of early intervention strategies. In: Proceedings of the North American Forest Insect Work Conference. Information Report NOR-X-381. Natural Resources Canada, Canadian Forest Service, Edmonton, AB, 2001. pp 57–68
Régnière J, Nealis V (2007) Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol Entomol 32:461–477
Reynolds RT, Scott JM, Nussbaum RA (1980) A variable circular-plot method for estimating bird numbers. Condor 82:309–313
Rimmer CC, Mcfarland KP (2012) Tennessee warbler (Oreothlypis peregrina). In: Poole A (ed) The birds of North America. Cornell Lab of Ornithology, Ithaca
Robitaille A, Saucier J (1998) Paysages régionaux du Québec meridional. Direction de la gestion des stocks forestiers et Direction des relations publiques, Ministère des Ressources Naturelles du Québec. Les publications du Québec, Québec
Rowe JS (1972) Forest regions of Canada Forest regions of Canada. Can. For. Serv. Publ. 1300
Royama T (1984) Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol Monogr 54:429–462
Sanders C (1970) Populations of breeding birds in the spruce-fir forests of Northwestern Ontario. Can F Nat 84:131–135
Smith MA, Eveleigh ES, McCann KS, Merilo MT, McCarthy PC, Van Rooyen KI (2011) Barcoding a quantified food web: crypsis, concepts, ecology and hypotheses. PLoS One 6:e14424
Speer JH, Swetnam TW, Wickman BE, Youngblood A (2001) Changes in pandora moth outbreak dynamics during the past 622 years. Ecology 82:679–697
Sturtevant BR, Cooke BJ, Kneeshaw DD, MacLean DA (2015) Modeling insect disturbance across forested landscapes: insights from the spruce budworm. In: Perera AH, Sturtevant BR, Buse LJ (eds) Simulation modeling of forest landscape disturbances. Springer, Cham, pp 93–134
Thériault S, Villard M-A, Haché SJBE (2012) Habitat selection in site-faithful ovenbirds and recruits in the absence of experimental attraction. Behav Ecol 23:1289–1295
Thibault M, Hotte D (1985) Les régions écologiques du Québec méridional: deuxième approximation. Ministère de l’énergie et des ressources, Service de la cartographie
Thomas L, Marques TA (2012) Passive acoustic monitoring for estimating animal density. Acou Today 8:35–44
Valone TJ, Templeton JJ (2002) Public information for the assessment of quality: a widespread social phenomenon. Phil Trans R Soc Lond B 357:1549–1557
Venier LA, Holmes SB (2010) A review of the interaction between forest birds and eastern spruce budworm. Environ Rev 18:191–207
Venier LA, Pearce JL, Fillman DR, McNicol DK, Welsh DA (2009) Effects of spruce budworm (Choristoneura fumiferana (Clem.)) outbreaks on boreal mixed-wood bird communities. Avian Conserv Ecol. https://doi.org/10.5751/ACE-00296-040103
Venier L, Holmes S, Williams J (2011) Bay-breasted warbler (Setophaga castanea). In: Poole A (ed) The birds of North America. Cornell Lab of Ornithology, Ithaca
Verboven N, Tinbergen JM, Verhulst S (2001) Food, reproductive success and multiple breeding in the great tit Parus major. Ardea 89:387–406
Virgin GVJ, MacLean DA, Kershaw JJA (2018) Topkill and stem defects initiated during an uncontrolled spruce budworm outbreak on Cape Breton Island, Nova Scotia. Forestry. 91:63–72
Walter JA, Firebaugh AL, Tobin PC, Haynes KJ (2016) Invasion in patchy landscapes is affected by dispersal mortality and mate-finding failure. Ecology 97:3389–3401
Wikelski M, Tertitski G (2016) Living sentinels for climate change effects. Science 352:775–776
Wimmer J, Towsey M, Roe P, Williamson I (2013) Sampling environmental acoustic recordings to determine bird species richness. Ecol Appl 23:1419–1428
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Yang LH, Bastow JL, Spence KO, Wright AN (2008) What can we learn from resource pulses. Ecology 89:621–634
Acknowledgements
We thank the Ministère des Forêts, de la Faune et des Parcs du Québec for giving us access to their aerial survey data on spruce budworm defoliation, and Y. Aubry for sharing avian point count data with us. We are also grateful to observers who performed bird point counts over the years. Funding was provided by a Strategic Partnership Grant for Projects from the Natural Sciences and Engineering Research Council of Canada to MAV, DK, PJ, JFP, and LDG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Germain, M., Kneeshaw, D., De Grandpré, L. et al. Insectivorous songbirds as early indicators of future defoliation by spruce budworm. Landscape Ecol 36, 3013–3027 (2021). https://doi.org/10.1007/s10980-021-01300-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-021-01300-z