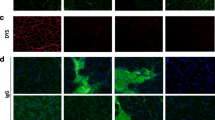
Abstract
Duchenne muscular dystrophy (DMD) is a degenerative skeletal muscle disease that makes walking and breathing difficult. DMD is caused by an X-linked (Xp21) mutation in the dystrophin gene. Dystrophin is a scaffolding protein located in the sarcolemmal cytoskeleton, important in maintaining structural integrity and regulating muscle cell (muscle fiber) growth and repair. Dystrophin deficiency in mouse models (e.g., mdx mouse) destabilizes the interface between muscle fibers and the extracellular matrix, resulting in profound damage, inflammation, and weakness in diaphragm and limb muscles. While the link between dystrophin deficiency with inflammation and pathology is multi-factorial, elevated oxidative stress has been proposed as a central mediator. Unfortunately, the use of non-specific antioxidant scavengers in mouse and human studies has led to inconsistent results, obscuring our understanding of the importance of redox signaling in pathology of muscular dystrophy. However, recent studies with more mechanistic approaches in mdx mice suggest that NAD(P)H oxidase and nuclear factor-kappaB are important in amplifying dystrophin-deficient muscle pathology. Therefore, more targeted antioxidant therapeutics may ameliorate damage and weakness in human population, thus promoting better muscle function and quality of life. This review will focus upon the pathobiology of dystrophin deficiency in diaphragm and limb muscle primarily in mouse models, with a rationale for development of targeted therapeutic antioxidants in DMD patients.

Similar content being viewed by others
References
Abdel-Salam E, Abdel-Meguid I, Korraa SS (2009) Markers of degeneration and regeneration in Duchenne muscular dystrophy. Acta Myol 28(3):94–100
Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC (2007) Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117(4):889–901
Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R (1999) Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol 33(4):959–965
Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC (2000) Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol 150(6):1385–1398
Allen DG, Gervasio OL, Yeung EW, Whitehead NP (2010) Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88(2):83–91
Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB (2007) Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol 102(3):956–964
Bach JR, Martinez D (2011) Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care 56(6):744–750
Badalamente MA, Stracher A (2000) Delay of muscle degeneration and necrosis in mdx mice by calpain inhibition. Muscle Nerve 23(1):106–111
Baker MS, Austin L (1989) The pathological damage in Duchenne muscular dystrophy may be due to increased intracellular oxy-radical generation caused by the absence of dystrophin and subsequent alterations in Ca2 + metabolism. Med Hypotheses 29(3):187–193
Berneske GM, Butson AR, Gauld EN, Levy D (1960) Clinical trial of high dosage vitamin E in human muscular dystrophy. Can Med Assoc J 82:418–421
Binder HJ, Herting DC, Hurst V, Finch SC, Spiro HM (1965) Tocopherol deficiency in man. N Engl J Med 273(24):1289–1297
Bornman L, Rossouw H, Gericke GS, Polla BS (1998) Effects of iron deprivation on the pathology and stress protein expression in murine X-linked muscular dystrophy. Biochem Pharmacol 56(6):751–757
Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS (1995) Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82(5):743–752
Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS (1996) Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84(5):757–767
Buck M, Chojkier M (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15(8):1753–1765
Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT (2002) Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J Clin Nutr 75(4):749–753
Cai B, Spencer MJ, Nakamura G, Tseng-Ong L, Tidball JG (2000) Eosinophilia of dystrophin-deficient muscle is promoted by perforin-mediated cytotoxicity by T cell effectors. Am J Pathol 156(5):1789–1796
Carlson CG, Samadi A, Siegel A (2005) Chronic treatment with agents that stabilize cytosolic IkappaB-alpha enhances survival and improves resting membrane potential in MDX muscle fibers subjected to chronic passive stretch. Neurobiol Dis 20(3):719–730
Carter GT, McDonald CM (2000) Preserving function in Duchenne dystrophy with long-term pulse prednisone therapy. Am J Phys Med Rehabil 79(5):455–458
Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA (2007) Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J 21(9):2195–2204
Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT (1996) Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci U S A 93(17):9142–9147
Chao DS, Silvagno F, Bredt DS (1998) Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J Neurochem 71(2):784–789
Chen M, Cheng C, Yan M, Niu S, Gao S, Shi S, Liu H, Qin Y, Shen A (2008) Involvement of CAPON and nitric oxide synthases in rat muscle regeneration after peripheral nerve injury. J Mol Neurosci 34(1):89–100
Childers MK, Okamura CS, Bogan DJ, Bogan JR, Petroski GF, McDonald K, Kornegay JN (2002) Eccentric contraction injury in dystrophic canine muscle. Arch Phys Med Rehabil 83(11):1572–1578
Crosbie RH, Straub V, Yun HY, Lee JC, Rafael JA, Chamberlain JS, Dawson VL, Dawson TM, Campbell KP (1998) mdx muscle pathology is independent of nNOS perturbation. Hum Mol Genet 7(5):823–829
Dalla Libera L, Sabbadini R, Renken C, Ravara B, Sandri M, Betto R, Angelini A, Vescovo G (2001) Apoptosis in the skeletal muscle of rats with heart failure is associated with increased serum levels of TNF-alpha and sphingosine. J Mol Cell Cardiol 33(10):1871–1878
De Pasquale L, D’Amico A, Verardo M, Petrini S, Bertini E, De Benedetti F (2012) Increased muscle expression of interleukin-17 in Duchenne muscular dystrophy. Neurology 78(17):1309–1314
Disatnik MH, Rando TA (1999) Integrin-mediated muscle cell spreading. The role of protein kinase c in outside-in and inside-out signaling and evidence of integrin cross-talk. J Biol Chem 274(45):32486–32492
Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, Rando TA (1998) Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci 161(1):77–84
Disatnik MH, Chamberlain JS, Rando TA (2000) Dystrophin mutations predict cellular susceptibility to oxidative stress. Muscle Nerve 23(5):784–792
Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, Ruegg UT (2006) Green tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol 290(2):C616–C625
Dupont-Versteegden EE, McCarter RJ (1992) Differential expression of muscular dystrophy in diaphragm versus hindlimb muscles of mdx mice. Muscle Nerve 15(10):1105–1110
Durham WJ, Arbogast S, Gerken E, Li YP, Reid MB (2006) Progressive nuclear factor-kappaB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve 34(3):298–303
Escolar DM, Scacheri CG (2001) Pharmacologic and genetic therapy for childhood muscular dystrophies. Curr Neurol Neurosci Rep 1(2):168–174
Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F (2006) Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J 20(6):705–707
Fenichel GM, Brooke MH, Griggs RC, Mendell JR, Miller JP, Moxley RT 3rd, Park JH, Provine MA, Florence J, Kaiser KK et al (1988) Clinical investigation in Duchenne muscular dystrophy: penicillamine and vitamin E. Muscle Nerve 11(11):1164–1168
Fisher AB (2009) Redox signaling across cell membranes. Antioxid Redox Signal 11(6):1349–1356
Galbiati F, Razani B, Lisanti MP (2001) Caveolae and caveolin-3 in muscular dystrophy. Trends Mol Med 7(10):435–441
Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG (2008) TRPC1 binds to caveolin-3 and is regulated by Src kinase - role in Duchenne muscular dystrophy. J Cell Sci 121(Pt 13):2246–2255
Grady RM, Grange RW, Lau KS, Maimone MM, Nichol MC, Stull JT, Sanes JR (1999) Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol 1(4):215–220
Grounds MD, Torrisi J (2004) Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J 18(6):676–682
Hartel JV, Granchelli JA, Hudecki MS, Pollina CM, Gosselin LE (2001) Impact of prednisone on TGF-beta1 and collagen in diaphragm muscle from mdx mice. Muscle Nerve 24(3):428–432
Hauser E, Hoger H, Bittner R, Widhalm K, Herkner K, Lubec G (1995) Oxyradical damage and mitochondrial enzyme activities in the mdx mouse. Neuropediatrics 26(5):260–262
Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D (1996) Oxidative damage to muscle protein in Duchenne muscular dystrophy. NeuroReport 8(1):357–361
Hess DR (2012) The growing role of noninvasive ventilation in patients requiring prolonged mechanical ventilation. Respir Care 57 (6):900–918; discussion 918–920
Hnia K, Gayraud J, Hugon G, Ramonatxo M, De La Porte S, Matecki S, Mornet D (2008) l-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am J Pathol 172(6):1509–1519
Hodgetts S, Radley H, Davies M, Grounds MD (2006) Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord 16(9–10):591–602
Hoshino S, Ohkoshi N, Ishii A, Shoji S (2002) The expression of alpha-dystrobrevin and dystrophin during skeletal muscle regeneration. J Muscle Res Cell Motil 23(2):131–138
Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L (2009) Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J 23(8):2539–2548
Ishikawa Y, Miura T, Aoyagi T, Ogata H, Hamada S, Minami R (2011) Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord 21(1):47–51
Jones KJ, Compton AG, Yang N, Mills MA, Peters MF, Mowat D, Kunkel LM, Froehner SC, North KN (2003) Deficiency of the syntrophins and alpha-dystrobrevin in patients with inherited myopathy. Neuromuscul Disord 13(6):456–467
Kaczor JJ, Hall JE, Payne E, Tarnopolsky MA (2007) Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic Biol Med 43(1):145–154
Kameya S, Miyagoe Y, Nonaka I, Ikemoto T, Endo M, Hanaoka K, Nabeshima Y, Takeda S (1999) Alpha1-syntrophin gene disruption results in the absence of neuronal-type nitric-oxide synthase at the sarcolemma but does not induce muscle degeneration. J Biol Chem 274(4):2193–2200
Kaminski HJ, Andrade FH (2001) Nitric oxide: biologic effects on muscle and role in muscle diseases. Neuromuscul Disord 11(6–7):517–524
Kim J-H, Lawler JM (2012) Amplification of proinflammatory phenotype, damage, and weakness by oxidative stress in the diaphragm muscle of mdx mice. Free Radic Biol Med 52(9):1597–1606
Kim J-H, Kwak HB, Lawler JM (2008) NAD(P)H oxidase inhibition upregulates anti-apoptotic BAG-4 protein expression in the mdx diaphragm. FASEB J 22(959):8
Kosek DJ, Bamman MM (2008) Modulation of the dystrophin-associated protein complex in response to resistance training in young and older men. J Appl Physiol 104(5):1476–1484
Kumar A, Boriek AM (2003) Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J 17(3):386–396
Kumar A, Takada Y, Boriek AM, Aggarwal BB (2004) Nuclear factor-kappaB: its role in health and disease. J Mol Med 82(7):434–448
Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D (2009) Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 119(3):624–635
Lawler JM, Song W, Demaree SR (2003) Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 35(1):9–16
Lawler JM, Hord JM, Yang Lee, Kumar Joshi, and Jong-Hee Kim (2011) Redox regulation of caveolin-3 and MMP-9 in the diaphragm of mdx mice. FASEB J LB:519
Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A (2009) Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet 18(14):2584–2598
Lim JH, Kim DY, Bang MS (2004) Effects of exercise and steroid on skeletal muscle apoptosis in the mdx mouse. Muscle Nerve 30(4):456–462
Lynch GS (2004) Role of contraction-induced injury in the mechanisms of muscle damage in muscular dystrophy. Clin Exp Pharmacol Physiol 31(8):557–561
Lynch GS, Rafael JA, Hinkle RT, Cole NM, Chamberlain JS, Faulkner JA (1997) Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol 272(6 Pt 1):C2063–C2068
Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA (2001) Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535(Pt 2):591–600
Matsumura K, Campbell KP (1994) Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve 17(1):2–15
McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y, Sugiura T, Powers SK (2007) Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med 175(2):150–159
Mendell JR, Engel WK, Derrer EC (1971) Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science 172(988):1143–1145
Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM (2010) Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med 363(15):1429–1437
Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G (2006a) Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol 168(3):918–926
Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, Squadrito F, Vita G (2006b) Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol 198(1):234–241
Messina S, Bitto A, Aguennouz M, Mazzeo A, Migliorato A, Polito F, Irrera N, Altavilla D, Vita GL, Russo M, Naro A, De Pasquale MG, Rizzuto E, Musaro A, Squadrito F, Vita G (2009) Flavocoxid counteracts muscle necrosis and improves functional properties in mdx mice: a comparison study with methylprednisolone. Exp Neurol 220(2):349–358
Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, Vita G (2011) Activation of NF-kappaB pathway in Duchenne muscular dystrophy: relation to age. Acta Myol 30(1):16–23
Miyagoe-Suzuki Y, Takeda SI (2001) Association of neuronal nitric oxide synthase (nNOS) with alpha1-syntrophin at the sarcolemma. Microsc Res Tech 55(3):164–170
Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G (2003) Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 60(6):993–997
Morrison J, Lu QL, Pastoret C, Partridge T, Bou-Gharios G (2000) T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest 80(6):881–891
Nakae Y, Stoward PJ, Kashiyama T, Shono M, Akagi A, Matsuzaki T, Nonaka I (2004) Early onset of lipofuscin accumulation in dystrophin-deficient skeletal muscles of DMD patients and mdx mice. J Mol Histol 35(5):489–499
Nakae Y, Hirasaka K, Goto J, Nikawa T, Shono M, Yoshida M, Stoward PJ (2008) Subcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: a quantitative histological, immunohistochemical and electrophysiological study. Histochem Cell Biol 129(4):489–501
Nguyen HX, Tidball JG (2003) Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol 553(Pt 3):833–841
Pan Y, Chen C, Shen Y, Zhu CH, Wang G, Wang XC, Chen HQ, Zhu MS (2008) Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol Cells 25(4):531–537
Partridge T (1991) Animal models of muscular dystrophy–what can they teach us? Neuropathol Appl Neurobiol 17(5):353–363
Pastoret C, Sebille A (1995) Age-related differences in regeneration of dystrophic (mdx) and normal muscle in the mouse. Muscle Nerve 18(10):1147–1154
Peters MF, Adams ME, Froehner SC (1997) Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol 138(1):81–93
Peterson JM, Guttridge DC (2008) Skeletal muscle diseases, inflammation, and NF-kappaB signaling: insights and opportunities for therapeutic intervention. Int Rev Immunol 27(5):375–387
Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH (2002) A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 11(3):263–272
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102(6):2389–2397
Ragusa RJ, Chow CK, Porter JD (1997) Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul Disord 7(6–7):379–386
Rando TA (2001a) The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve 24(12):1575–1594
Rando TA (2001b) Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc Res Tech 55(4):223–235
Rando TA (2002) Oxidative stress and the pathogenesis of muscular dystrophies. Am J Phys Med Rehabil 81(11 Suppl):S175–S186
Rando TA, Disatnik MH, Yu Y, Franco A (1998) Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul Disord 8(1):14–21
Rodriguez MC, Tarnopolsky MA (2003) Patients with dystrophinopathy show evidence of increased oxidative stress. Free Radic Biol Med 34(9):1217–1220
Roelofs RI, de Arango GS, Law PK, Kinsman D, Buchanan DC, Park JH (1979) Treatment of Duchenne’s muscular dystrophy with penicillamine. Results of a double-blind trial. Arch Neurol 36(5):266–268
Sandri M, Carraro U (1999) Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol 31(12):1373–1390
Selsby JT (2011) Increased catalase expression improves muscle function in mdx mice. Exp Physiol 96(2):194–202
Shiao T, Fond A, Deng B, Wehling-Henricks M, Adams ME, Froehner SC, Tidball JG (2004) Defects in neuromuscular junction structure in dystrophic muscle are corrected by expression of a NOS transgene in dystrophin-deficient muscles, but not in muscles lacking alpha- and beta1-syntrophins. Hum Mol Genet 13(17):1873–1884
Skrabek RQ, Anderson JE (2001) Metabolic shifts and myocyte hypertrophy in deflazacort treatment of mdx mouse cardiomyopathy. Muscle Nerve 24(2):192–202
Spencer MJ, Tidball JG (2001) Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord 11(6–7):556–564
Spencer MJ, Walsh CM, Dorshkind KA, Rodriguez EM, Tidball JG (1997) Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest 99(11):2745–2751
Spencer MJ, Marino MW, Winckler WM (2000) Altered pathological progression of diaphragm and quadriceps muscle in TNF-deficient, dystrophin-deficient mice. Neuromuscul Disord 10(8):612–619
Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG (2001) Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 98(2):235–243
Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP (2008) Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord 18(5):371–381
Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352(6335):536–539
Stern LZ, Ringel SP, Ziter FA, Menander-Huber KB, Ionasescu V, Pellegrino RJ, Snyder RD (1982) Drug trial of superoxide dismutase in Duchenne’s muscular dystrophy. Arch Neurol 39(6):342–346
Stevens ED, Faulkner JA (2000) The capacity of mdx mouse diaphragm muscle to do oscillatory work. J Physiol 522(Pt 3):457–466
Sunada Y, Ohi H, Hase A, Hosono T, Arata S, Higuchi S, Matsumura K, Shimizu T (2001) Transgenic mice expressing mutant caveolin-3 show severe myopathy associated with increased nNOS activity. Hum Mol Genet 10(3):173–178
Sussman M (2002) Duchenne muscular dystrophy. J Am Acad Orthop Surg 10(2):138–151
Tidball JG, Wehling-Henricks M (2004) Expression of a NOS transgene in dystrophin-deficient muscle reduces muscle membrane damage without increasing the expression of membrane-associated cytoskeletal proteins. Mol Genet Metab 82(4):312–320
Tidball JG, Wehling-Henricks M (2007) The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol 102(4):1677–1686
Tkatchenko AV, Le Cam G, Léger JJ, Dechesne CA (2000) Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochim Biophys Acta 1500 (1):17–30
Ushio-Fukai M (2009) Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11(6):1289–1299
Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ (2006) Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5(2):109–117
Venema VJ, Ju H, Zou R, Venema RC (1997) Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 272(45):28187–28190
Wakayama Y, Inoue M, Murahashi M, Shibuya S, Jimi T, Kojima H, Oniki H (1997) Ultrastructural localization of alpha 1-syntrophin and neuronal nitric oxide synthase in normal skeletal myofiber, and their relation to each other and to dystrophin. Acta Neuropathol 94(5):455–464
Walton JN, Nattrass FJ (1954) On the classification, natural history and treatment of the myopathies. Brain 77(2):169–231
Warren GL, Hayes DA, Lowe DA, Prior BM, Armstrong RB (1993) Materials fatigue initiates eccentric contraction-induced injury in rat soleus muscle. J Physiol 464:477–489
Wehling M, Spencer MJ, Tidball JG (2001) A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol 155(1):123–131
Wehling-Henricks M, Sokolow S, Lee JJ, Myung KH, Villalta SA, Tidball JG (2008) Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet 17(15):2280–2292
Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG (2006) Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord 16(12):845–854
Whitehead NP, Pham C, Gervasio OL, Allen DG (2008) N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 586(7):2003–2014
Whitehead NP, Yeung EW, Froehner SC, Allen DG (2010) Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS ONE 5(12):e15354
Williams IA, Allen DG (2007) The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 293(3):H1969–H1977
Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP (1997) Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet 6(6):831–841
Yiu EM, Kornberg AJ (2008) Duchenne muscular dystrophy. Neurol India 56(3):236–247
Yoshida M, Hama H, Ishikawa-Sakurai M, Imamura M, Mizuno Y, Araishi K, Wakabayashi-Takai E, Noguchi S, Sasaoka T, Ozawa E (2000) Biochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy. Hum Mol Genet 9(7):1033–1040
Zhou L, Lu H (2010) Targeting fibrosis in Duchenne muscular dystrophy. J Neuropathol Exp Neurol 69(8):771–776
Acknowledgments
This work is supported by the Sydney and JL Huffines research grant (JHK, JML) and NIH (AG017768, AR054084) (LVT, JML).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, JH., Kwak, HB., Thompson, L.V. et al. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J Muscle Res Cell Motil 34, 1–13 (2013). https://doi.org/10.1007/s10974-012-9330-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-012-9330-9