Abstract
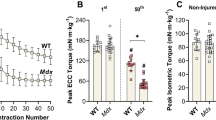
X-linked muscular dystrophy of the mouse (mdx) has been reported to progressively remodel skeletal muscle to preferentially reduce fast fiber composition. Despite this, mdx muscle displays normal levels of posttetanic potentiation (PTP). Since PTP may primarily depend on phosphorylation of the myosin regulatory light chain (RLC) in fast muscle fibers, maintenance of PTP with mdx disease progression is paradoxical and may represent an adaptation of the diseased muscle. This study assesses the role of RLC phosphorylation during PTP of mdx muscle. Extensor digitorum longus muscles were isolated from mdx and from C57BL/10 (control) mice at ~50 (young) and ~300 (adult) days and stimulated in vitro (25°C) to induce PTP. During potentiation, muscles were harvested for subsequent determination of RLC phosphorylation levels. Immunofluorescence was used to assess muscle fiber type composition and no age effects were found. The magnitude of PTP was higher (P < 0.05) in mdx than control muscles at both young (mdx: 21.9 ± 1.6%; control: 17.7 ± 1.2%) and adult (mdx: 30.4 ± 1.8%; control: 23.2 ± 2.2%) ages. However, RLC phosphate content was similar between all groups both at rest and following stimulation. Our results are consistent with a model where the sensitivity of mdx muscle to RLC phosphorylation-induced force potentiation is increased by disease- and age-dependent alterations in excitation-contraction coupling noted for mdx and aging muscle.





Similar content being viewed by others
Abbreviations
- ½RT:
-
Half relaxation time
- AC:
-
Adult control
- AM:
-
Adult mdx
- CS:
-
Conditioning stimulus
- CSA:
-
Cross sectional area
- EDL:
-
Extensor digitorum longus
- ICT:
-
Intracellular calcium transient
- Lo :
-
Optimal length for twitch tension development
- mdx :
-
X-linked muscular dystrophy
- MHC:
-
myosin heavy chain
- PTP:
-
Posttetanic potentiation
- RLC:
-
Regulatory light chain
- skMLCK:
-
Skeletal muscle isoform of myosin light chain kinase
- TPT:
-
Time to peak tension
- YC:
-
Young control
- YM:
-
Young mdx
References
Anderson JE, Bressler BH, Ovalle WK (1988) Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil 9:499–515
Brown IA, Loeb GE (1998) Post-activation potentiation: a clue for simplifying models of muscle dynamics. Am Zool 38:743–754
Bulfield G, Siller WG, Wight PAL, Moore KJ (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81:1189–1192
Close R, Hoh JF (1969) Post-tetanic potentiation of twitch contractions of cross-innervated rat fast and slow muscles. Nature 221:179–181
Coirault C, Lambert F, Marchand-Adam S, Attal P, Chemla D, Lecarpentier Y (1999) Myosin molecular motor dysfunction in dystrophic mouse diaphragm. Am J Physiol 277:C1170–C1176
Coirault C, Lambert F, Pourny JC, Lecarpentier Y (2002) Velocity of actomyosin sliding in vitro is reduced in dystrophic mouse diaphragm. Am J Respir Crit Care Med 165(2):250–253
DiMario JX, Uzman A, Strohman RC (1991) Fiber regeneration is not persistent in dystrophic (mdx) mouse skeletal muscle. Dev Biol 148:314–321
González E, Messi ML, Zheng Z, Delbono O (2003) Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol 552:833–844
Hayes A, Williams DA (1996) Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol 80:670–679
Hollingworth S, Zeiger U, Baylor SM (2008) Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J Physiol 586:5063–5075
Hopf FW, Turner PR, Denetclaw WF, Reddy P, Steinhardt RA (1996) A critical evaluation of resting intracellular free calcium regulation in dystrophic mdx muscle. Am J Physiol 271:C1325–C1339
Kamm KE, Hsu LC, Kubota Y, Stull JT (1989) Phosphorylation of smooth muscle myosin heavy and light chains. J Biol Chem 264:21223–21229
Klug GA, Botterman BR, Stull JT (1982) The effect of low frequency stimulation on myosin light chain phosphorylation in skeletal muscle. J Biol Chem 257:4688–4690
Krarup C (1981) Enhancement and diminution of mechanical tension evoked by staircase and by tetanus in rat muscle. J Physiol 311:355–372
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lamb GD, Junankar PR, Stephenson DG (1995) Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscles of rat and toad. J Physiol 489:349–362
Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA (2008) Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve 38(4):1290–1303
Lännergren J, Westerblad H (1987) The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol 390:285–293
Lännergren J, Bruton JD, Westerblad H (2000) Vacuole formation in fatigued skeletal muscle fibres from frog and mouse: effects of extracellular lactate. J Physiol 526:597–611
Louboutin JP, Fichter-Gagnepain V, Thaon E, Fardeau M (1993) Morphometric analysis of mdx diaphram muscle fibres. Comparison with hindlimb muscles. Neuromuscul Disord 3(5–6):463–469
Louboutin JP, Fichter-Gagnepain V, Pastoret C, Thaon E, Noireaud J, Sébille A, Fardeau M (1995) Morphological and functional study of extensor digitorum longus muscle regeneration after iterative crush lesions in mdx muscle. Neuromuscul Disord 5:489–500
Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA (2001) Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535:591–600
Mallouk N, Jacquemond V, Allard B (2000) Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K(+) channels. Proc Natl Acad Sci USA 97:4950–4955
Manning DR, Stull JT (1982) Myosin light chain phosphorylation-dephosphorylation in mammalian skeletal muscle. Am J Physiol 242:C234–C241
Mechalchuk CL, Bressler BH (1992) Contractility of mdx skeletal muscle after denervation and devascularization. Muscle Nerve 15:310–317
Mendez J, Keys A (1960) Density and composition of mammalian muscle. Metabolism 9:184–199
Moore RL, Stull JT (1984) Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am J Physiol 247:C462–C471
Moore RL, Palmer BM, Williams SL, Tanabe H, Grange RW, Houston ME (1990) Effect of temperature on myosin phosphorylation in mouse skeletal muscle. Am J Physiol 259:C432–C438
Palmer B, Moore R (1989) Myosin light chain phosphorylation and tension potentiation in mouse skeletal muscle. Am J Physiol 257:C1012–C1019
Payne AM, Jiménez-Moreno R, Wang ZM, Messi ML, Delbono O (2009) Role of Ca2+, membrane excitability, and Ca2+ stores in failing muscle contraction with aging. Exp Gerontol 44:261–273
Persechini A, Stull JT, Cooke R (1985) The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem 260:7951–7954
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993a) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90:3710–3714
Petrof BJ, Stedman HH, Shrager JB, Eby J, Sweeney HL, Kelly AM (1993b) Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol 265:C834–C841
Quinlan JG, Johnson SR, McKee MK, Lyden SP (1992) Twitch and tetanus in mdx mouse muscle. Muscle Nerve 15:837–842
Ribaux P, Bleicher F, Couble ML, Amsellem J, Cohen SA, Berthier C, Blaineau S (2001) Voltage-gated sodium channel (SkM1) content in dystrophin-deficient muscle. Pflugers Arch 441:746–755
Rousseau J, Dumont N, Lebel C, Quenneville SP, Côté CH, Frenette J, Tremblay JP (2010) Dystrophin expression following the transplantation of normal muscle precursor cells protects mdx muscle from contraction induced damage. Cell Transplant 19(5):589–596
Ryder JW, Lau KS, Kamm KE, Stull JT (2007) Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase. J Biol Chem 282:20447–20454
Sacco P, Jones DA, Dick JR, Vrbova G (1992) Contractile properties and susceptibility to exercise-induced damage of normal and mdx mouse tibialis anterior muscle. Clin Sci (Lond) 82:227–236
Schacterle GR, Pollack RL (1973) A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem 51:654–655
Sweeney HL, Stull JT (1986) Phosphorylation of myosin in permeablized mammalian cardiac and skeletal muscle cells. Am J Physiol 250:C657–C660
Sweeney HL, Bowman BM, Stull JT (1993) Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol 264:C1085–C1095
Turner PR, Westwood T, Regen CM, Steinhardt RA (1988) Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nat Lond 335:735–738
Vandenboom R, Xeni J, Bestic NM, Houston ME (1997) Increased force development rates of fatigued mouse skeletal muscle are graded to myosin light chain phosphate content. Am J Physiol 272:R1980–R1984
Wang ZM, Messi ML, Delbono O (2000) L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J 78:1947–1954
Wolff AV, Niday AK, Voelker KA, Call JA, Evans NP, Granata KP, Grange RW (2006) Passive mechanical properties of maturing extensor digitorum longus are not affected by lack of dystrophin. Muscle Nerve 34:304–312
Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT (2005) Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci USA 102:17519–17524
Acknowledgments
This study was supported by funds provided by the Natural Sciences and Engineering Research Council of Canada. I.C. Smith was funded through a Masters Studentship from the Canadian Institutes of Health Research. We would like to thank Dr. Jim Stull for his assistance in the analysis of the myosin RLC phosphate content.
Conflict of interest
The authors do not have any conflicting interests regarding the findings or interpretations of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, I.C., Huang, J., Quadrilatero, J. et al. Posttetanic potentiation in mdx muscle. J Muscle Res Cell Motil 31, 267–277 (2010). https://doi.org/10.1007/s10974-010-9229-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-010-9229-2