Abstract
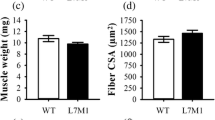
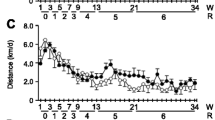
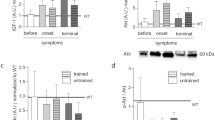
Myostatin-deficient mice (MSTN −/−) display excessive muscle mass and this is associated with a profound loss of oxidative metabolic properties. In this study we analysed the effect of two endurance-based exercise regimes, either a forced high-impact swim training or moderate intensity voluntary wheel running on the adaptive properties of the tibialis anterior and plantaris muscle from MSTN −/− mice. MSTN −/− and wild type (MSTN +/+) animals had comparable performances in the wheel running regime in terms of distance, average speed and time, but MSTN −/− mice showed a reduced ability to sustain a high-impact activity via swimming. Swim training elicited muscle specific adaptations on fibre type distribution in MSTN −/−; the tibialis anterior displaying a partial transformation in contrast to the plantaris which showed no change. Conversely, wheel running induced similar changes in fibre type composition of both muscles, favouring transitions from IIB-to-IIA. Succinate dehydrogenase activity, an indicator of mitochondrial oxidative potential was increased in response to either exercise regime, with wheel running eliciting more robust changes in the MSTN −/− muscles. Examination of the cross sectional area of individual fibre types showed genotype-specific responses with MSTN −/− mice exhibiting an incapability of fibre enlargement following the wheel running regime, as opposed to MSTN +/+ mice and a greater susceptibility to muscle fibre area loss following swimming. In conclusion, the muscle fibre hypertrophy, oxidative capacity and glycolytic phenotype of myostatin deficient muscle can be altered with endurance exercise regimes.






Similar content being viewed by others
References
Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA (2001a) Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90:1900–1908
Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA (2001b) Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol 280:C637–C645
Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K (2007) Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA 104:1835–1840
Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T (2009) Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci USA 106:7479–7484
Billat LV (1996) Use of blood lactate measurements for prediction of exercise performance and for control of training Recommendations for long-distance running. Sports Med 22:157–175
Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C (2009) Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23:3896–3905
de Leon R, Hodgson JA, Roy RR, Edgerton VR (1994) Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res 654:241–250
Dimauro J, Balnave RJ, Shorey CD (1992) Effects of anabolic steroids and high intensity exercise on rat skeletal muscle fibres and capillarization A morphometric study. Eur J Appl Physiol Occup Physiol 64:204–212
Elashry MI, Otto A, Matsakas A, El-Morsy SE, Patel K (2009) Morphology and myofiber composition of skeletal musculature of the forelimb in young and aged wild type and myostatin null mice. Rejuvenation Res 12:269–281
Gruner JA, Altman J (1980) Swimming in the rat: analysis of locomotor performance in comparison to stepping. Exp Brain Res 40:374–382
Gulve EA, Rodnick KJ, Henriksen EJ, Holloszy JO (1993) Effects of wheel running on glucose transporter (GLUT4) concentration in skeletal muscle of young adult and old rats. Mech Ageing Dev 67:187–200
Hamrick MW, Samaddar T, Pennington C, McCormick J (2006) Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res 21:477–483
Hayes A, Lynch GS, Williams DA (1993) The effects of endurance exercise on dystrophic mdx mice I. Contractile and histochemical properties of intact muscles. Proc Biol Sci 253:19–25
Holmes JH, Ashmore CR, Robinson DW (1973) Effects of stress on cattle with hereditary muscular hypertrophy. J Anim Sci 36:684–694
Houle-Leroy P, Garland T Jr, Swallow JG, Guderley H (2000) Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol 89:1608–1616
Ikeda S, Kawamoto H, Kasaoka K, Hitomi Y, Kizaki T, Sankai Y, Ohno H, Haga S, Takemasa T (2006) Muscle type-specific response of PGC-1 alpha and oxidative enzymes during voluntary wheel running in mouse skeletal muscle. Acta Physiol (Oxf) 188:217–223
Ishihara A, Hirofuji C, Nakatani T, Itoh K, Itoh M, Katsuta S (2002) Effects of running exercise with increasing loads on tibialis anterior muscle fibres in mice. Exp Physiol 87:113–116
Jasmin BJ, Gardiner PF (1987) Patterns of EMG activity of rat plantaris muscle during swimming and other locomotor activities. J Appl Physiol 63:713–718
Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA (2005) Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol 289:H455–H465
Kovanen V, Suominen H, Heikkinen E (1980) Connective tissue of “fast” and “slow” skeletal muscle in rats–effects of endurance training. Acta Physiol Scand 108:173–180
Laughlin MH, Mohrman SJ, Armstrong RB (1984) Muscular blood flow distribution patterns in the hindlimb of swimming rats. Am J Physiol 246:H398–H403
Lennon DL, Mance MJ (1986) Interorgan cooperativity in carnitine metabolism in the trained state. J Appl Physiol 60:1659–1664
Lynch GS, Stephenson DG, Williams DA (1991) Endurance exercise effects on the contractile properties of single, skinned skeletal muscle fibres of young rats. Pflugers Arch 418:161–167
Matsakas A, Patel K (2009) Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol Histopathol 24:611–629
Matsakas A, Nikolaidis MG, Kokalas N, Mougios V, Diel P (2004) Effect of voluntary exercise on the expression of IGF-I and androgen receptor in three rat skeletal muscles and on serum IGF-I and testosterone levels. Int J Sports Med 25:502–508
Matsakas A, Friedel A, Hertrampf T, Diel P (2005) Short-term endurance training results in a muscle-specific decrease of myostatin mRNA content in the rat. Acta Physiol Scand 183:299–307
Matsakas A, Bozzo C, Cacciani N, Caliaro F, Reggiani C, Mascarello F, Patruno M (2006) Effect of swimming on myostatin expression in white and red gastrocnemius muscle and in cardiac muscle of rats. Exp Physiol 91:983–994
Matsakas A, Foster K, Otto A, Macharia R, Elashry MI, Feist S, Graham I, Foster H, Yaworsky P, Walsh F, Dickson G, Patel K (2009) Molecular, cellular and physiological investigation of myostatin propeptide-mediated muscle growth in adult mice. Neuromuscul Disord 19:489–499
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461
McPherron AC, Lee SJ (2002) Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109:595–601
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Mendias CL, Marcin JE, Calerdon DR, Faulkner JA (2006) Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol 101:898–905
Nakao C, Ookawara T, Kizaki T, Oh-Ishi S, Miyazaki H, Haga S, Sato Y, Ji LL, Ohno H (2000) Effects of swimming training on three superoxide dismutase isoenzymes in mouse tissues. J Appl Physiol 88:649–654
Pearson KG, Acharya H, Fouad K (2005) A new electrode configuration for recording electromyographic activity in behaving mice. J Neurosci Methods 148:36–42
Peijie C, Zicai D, Haowen X, Renbao X (2004) Effects of chronic and acute training on glucocorticoid receptors concentrations in rats. Life Sci 75:1303–1311
Petridou A, Nikolaidis MG, Matsakas A, Schulz T, Michna H, Mougios V (2005) Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle, and adipose tissue. Eur J Appl Physiol 94:84–92
Pette D (2002) The adaptive potential of skeletal muscle fibers. Can J Appl Physiol 27:423–448
Pette D, Staron RS (2001) Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol 115:359–372
Rehfeldt C, Ott G, Gerrard DE, Varga L, Schlote W, Williams JL, Renne U, Bunger L (2005) Effects of the compact mutant myostatin allele Mstn (Cmpt-dl1Abc) introgressed into a high growth mouse line on skeletal muscle cellularity. J Muscle Res Cell Motil 26:103–112
Roy RR, Hirota WK, Kuehl M, Edgerton VR (1985) Recruitment patterns in the rat hindlimb muscle during swimming. Brain Res 337:175–178
Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR (1991) EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol 70:2522–2529
Simoneau JA, Pette D (1988) Species-specific effects of chronic nerve stimulation upon tibialis anterior muscle in mouse, rat, guinea pig, and rabbit. Pflugers Arch 412:86–92
Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M (2006) Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol 209:866–873
Yancey SL, Overton JM (1993) Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol 75:1334–1340
Acknowledgments
K.P. and A.M would like to thank the Wellcome Trust (078649) and University of Reading and H.A. and E.M. acknowledge the Association Française contre les Myopathies for generous funding permitting the execution of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Matsakas and E. Mouisel contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsakas, A., Mouisel, E., Amthor, H. et al. Myostatin knockout mice increase oxidative muscle phenotype as an adaptive response to exercise. J Muscle Res Cell Motil 31, 111–125 (2010). https://doi.org/10.1007/s10974-010-9214-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-010-9214-9