Abstract
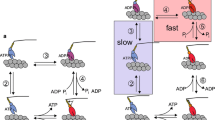
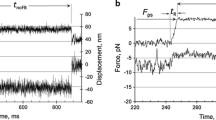
The ATPases (±Ca2+) of myofibrils from rabbit soleus (a slow muscle) and psoas (a fast muscle) have different E a: −Ca2+, 78 and 60 kJ/mol and +Ca2+, 155 and 71 kJ/mol, respectively. At physiological temperatures, the two types of myofibrillar ATPase are very similar and yet the mechanical properties of the muscles are different (Candau et al. (2003) Biophys J 85: 3132–3141). Muscle contraction relies on specific interactions of the different chemical states on the myosin head ATPase pathway with the thin filament. An explanation for the E a data is that different states populate the pathways of the two types of myofibril because the rate limiting steps are different. Here, we put this to the test by a comparison of the transient kinetics of the initial steps of the ATPases of the two types of myofibril at 4°C. We used two methods: rapid flow quench (`cold ATP chase': titration of active sites, ATP binding kinetics, k cat; `Pi burst': ATP cleavage kinetics) and fluorescence stopped-flow (MDCC-phosphate binding protein for free Pi; myofibrillar tryptophan fluorescence for myosin head-thin filament detachment and ATP cleavage kinetics). We find that, as with psoas myofibrils, the most populated state on the cross-bridge cycle of soleus myofibrils, whether relaxed or activated, is (A)M·ADP·Pi. We propose a reaction pathway that includes several (A)M·ADP·Pi sub-states that are either `weak' or `strong', depending on the mechanical condition.
Similar content being viewed by others
References
Bagshaw CR and Trentham DR (1973) The reversibility of adenosine triphosphate cleavage by myosin. Biochem J 133: 323–328.
Bagshaw CR and Trentham DR (1974) The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J 141: 331–349.
Barman T, Brune M, Lionne C, Piroddi N, Poggesi C, Stehle R, Tesi C, Travers F and Webb MR (1998) ATPase and shortening rates in frog fast skeletal myofibrils by time-resolved measurements of protein-bound and free Pi. Biophys J 74: 3120–3130.
Barman TE and Travers F (1985) The rapid-flow-quench method in the study of fast reactions in biochemistry: extension to subzero conditions. Meth. Biochem Anal 31: 1–59.
Biosca JA, Travers F, Hillaire D and Barman TE (1984) Cryoenzymic studies on myosin subfragment 1: perturbation of an enzyme reaction by temperature and solvent. Biochemistry 23: 1947–1955.
Biosca JA, Greene LE and Eisenberg E (1988) Binding of ADP and 5'-adenylyl imidodiphosphate to rabbit muscle myofibrils. J Biol Chem 263: 14231–14235.
Bottinelli R and Reggiani C (2000) Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73: 195–262.
Bremel RD and Weber A (1972) Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol 238: 97–101.
Brune M, Hunter JL, Corrie JE and Webb MR (1994) Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry 33: 8262–8271.
Candau R, Iorga B, Travers F, Barman T and Lionne C (2003) At physiological temperatures the ATPase rates of shortening soleus and psoas myofibrils are similar. Biophys J 85: 3132–3141.
Chaussepied P, Mornet D and Kassab R (1986) Identification of polyphosphate recognition sites communicating with actin sites on the skeletal myosin subfragment 1 heavy chain. Biochemistry 25: 6426–6432.
Cooke R (1997) Actomyosin interaction in striated muscle. Physiol Rev 77: 671–697.
Dantzig JA, Goldman YE, Millar NC, Lacktis J and Homsher E (1992) Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol 451: 247–278.
Fitzsimons DP, Patel JR, Campbell KS and Moss RL (2003) Effect of phosphate on loaded shortening in skeletal muscle fibers. Biophys J 84: 246a.
Geeves MA (1991) The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J 274: 1–14.
Geeves MA and Holmes KC (1999) Structural mechanism of muscle contraction. Annu Rev Biochem 68: 687–728.
Geeves MA and Trentham DR (1982) Protein-bound adenosine 5'-triphosphate: properties of a key intermediate of the magnesiumdependent subfragment 1 adenosinetriphosphatase from rabbit skeletal muscle. Biochemistry 21: 2782–2789.
Goldman YE (1987) Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev Physiol 49: 637–654.
Goody RS (2003) The missing link in the muscle cross-bridge cycle. Nat Struct Biol 10: 773–775.
Han YS, Geiger PC, Cody MJ, Macken RL and Sieck GC (2003) ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol 94: 2188–2196.
He Z, Stienen GJ, Barends JP and Ferenczi MA (1998) Rate of phosphate release after photoliberation of adenosine 5¢-triphosphate in slow and fast skeletal muscle fibers. Biophys J 75: 2389–2401.
He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA and Reggiani C (2000) ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 79: 945–961.
Herrmann C, Houadjeto M, Travers F and Barman T (1992) Early steps of the Mg2+-ATPase of relaxed myofibrils. A comparison with Ca2+-activated myofibrils and myosin subfragment 1. Biochemistry 31: 8036–8042.
Herrmann C, Lionne C, Travers F and Barman T (1994) Correlation of ActoS1, myofibrillar, and muscle fiber ATPases. Biochemistry 33: 4148–4154.
Holmes KC, Angert I, Kull FJ, Jahn W and Schroder RR (2003) Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature 425: 423–427.
Houadjeto M, Travers F and Barman T (1992) Ca2+-activated myofibrillar ATPase: transient kinetics and the titration of its active sites. Biochemistry 31: 1564–1569.
Huxley AF (1980) Reflections on Muscle. Liverpool University Press, Liverpool.
Kawai M (2003) What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres? J Muscle Res Cell Motil 24: 127–138.
Lionne C, Brune M, Webb MR, Travers F and Barman T (1995) Time resolved measurements show that phosphate release is the rate limiting step on myofibrillar ATPases. FEBS Lett 364: 59–62.
Lionne C, Stehle R, Travers F and Barman T (1999) Cryoenzymic studies on an organized system: myofibrillar ATPases and shortening. Biochemistry 38: 8512–8520.
Lionne C, Iorga B, Candau R, Piroddi N, Webb MR, Belus A, Travers F and Barman T (2002) Evidence that phosphate release is the ratelimiting step on the overall ATPase of psoas myofibrils prevented from shortening by chemical cross-linking. Biochemistry 41: 13297–13308.
Lionne C, Iorga B, Candau R and Travers F (2003) Why choose myofibrils to study muscle myosin ATPase? J Muscle Res Cell Motil 24: 139–148.
Lymn RW and Taylor EW (1971) Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10: 4617–4624.
Málnási-Csizmadia A, Woolley RJ and Bagshaw CR (2000) Resolution of conformational states of Dictyostelium myosin II motor domain using tryptophan (W501) mutants: implications for the open-closed transition identified by crystallography. Biochemistry 39: 16135–16146.
Pate E and Cooke R (1989) A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil 10: 181–196.
Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC and Milligan RA (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261: 58–65.
Reimann EM and Umfleet RA (1978) Selective precipitation of 32Pi onto filter papers. Application to ATPase and cyclic AMP phosphodiesterase determination. Biochim Biophys Acta 523: 516–521.
Reubold TF, Eschenburg S, Becker A, Kull FJ and Manstein DJ (2003) A structural model for actin-induced nucleotide release in myosin. Nat Struct Biol 10: 826–830.
Rosenfeld SS and Taylor EW (1984) The ATPase mechanism of skeletal and smooth muscle acto-subfragment 1. J Biol Chem 259: 11908–11919.
Schaub MC, Watterson JG, Loth K and Foletta D (1983) The role of magnesium in binding of the nucleotide polyphosphate chain to the active site of myosin subfragment-1. Eur J Biochem 134: 197–204.
Siemankowski RF, Wiseman MO and White HD (1985) ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA 82: 658–662.
Stehle R, Lionne C, Travers F and Barman T (2000) Kinetics of the initial steps of rabbit psoas myofibrillar ATPases studied by tryptophan and pyrene fluorescence stopped-flow and rapid flowquench. Evidence that cross-bridge detachment is slower than ATP binding. Biochemistry 39: 7508–7520.
Sutoh K and Harrington WF (1977) Cross-linking of myosin thick filaments under activating and rigor conditions. A study of the radial disposition of cross-bridges. Biochemistry 16: 2441–2449.
Tesi C, Bachouchi N, Barman T and Travers F (1989) Cryoenzymic studies on myosin: transient kinetic evidence for two types of head with different ATP binding properties. Biochimie 71: 363–372.
Tesi C, Colomo F, Nencini S, Piroddi N and Poggesi C (2000) The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys J 78: 3081–3092.
Tesi C, Colomo F, Piroddi N and Poggesi C (2002) Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J Physiol 541: 187–199.
Tikunov BA, Sweeney HL and Rome LC (2001) Quantitative electrophoretic analysis of myosin heavy chains in single muscle fibers. J Appl Physiol 90: 1927–1935.
Wang G and Kawai M (1996) Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibers. Biophys J 71: 1450–1461.
Weiss S, Rossi R, Pellegrino MA, Bottinelli R and Geeves MA (2001) Differing ADP release rates from myosin heavy chain isoforms define the shortening velocity of skeletal muscle fibers. J Biol Chem 276: 45902–45908.
White HD, Belknap B and Webb MR (1997) Kinetics of nucleoside triphosphate cleavage and phosphate release steps by associated rabbit skeletal actomyosin, measured using a novel fluorescent probe for phosphate. Biochemistry 36: 11828–11836.
Yates LD and Greaser ML (1983) Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol 168: 123–141.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iorga, B., Candau, R., Travers, F. et al. Does phosphate release limit the ATPases of soleus myofibrils? Evidence that (A)M· ADP·Pi states predominate on the cross-bridge cycle. J Muscle Res Cell Motil 25, 367–378 (2004). https://doi.org/10.1007/s10974-004-0812-2
Issue Date:
DOI: https://doi.org/10.1007/s10974-004-0812-2