Abstract
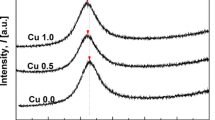
We have prepared the metastable solid solutions of (Fe2O3)1−x(Al2O3)x (x = 0.25, 0.50, and 0.75) with the corundum-type structure through the mechanical alloying method and measured the low-temperature heat-capacity below 300 K using the Physical Property Measurement System (PPMS), which have not been measured so far since the solid solution is not available through the conventional synthesis procedure. The measured heat capacities exhibit similar general trends of temperature dependence with the pure α-Fe2O3 and α-Al2O3 listed in the JANAF thermodynamic table, while the measured values are a little higher than that of their weighted averages. This might be due to the defects or lattice distortion introduced at the preparation using the mechanical alloying method. Moreover, heat capacity anomaly was observed at low-temperature regions below 25 K, which grows larger and shifts toward the higher temperature with increasing aluminum substitution, which might be caused by the unexpected phase transition or point defects of cations.





Similar content being viewed by others
References
Du J, Li Q, Chai J, Jiang L, Zhang Q, Han N, Zhang W, Tang B. Review of metal oxides as anode materials for lithium-ion batteries. Dalton Trans. 2022;51(25):9584–90. https://doi.org/10.1039/d2dt01415g.
Nzereogu PU, Omah AD, Ezema FI, Iwuoha EI, Nwanya AC. Anode materials for lithium-ion batteries: a review. Appl Surface Sci Adv. 2022;9: 100233. https://doi.org/10.1016/j.apsadv.2022.100233.
Fang S, Bresser D, Passerini S. Transition metal oxide anodes for electrochemical energy storage in lithium‐ and sodium‐ion batteries. Adv Energy Mater. 2019;10(1). https://doi.org/10.1002/aenm.201902485.
Lin Y-M, Abel PR, Heller A, Mullins CB. Α-Fe2O3 nanorods as anode material for lithium ion batteries. J Phys Chem Lett. 2011;2(22):2885–91. https://doi.org/10.1021/jz201363j.
Wang Q, Guo C, He J, Yang S, Liu Z, Wang Q. Fe2O3/c-modified si nanoparticles as anode material for high-performance lithium-ion batteries. J Alloy Compd. 2019;795:284–90. https://doi.org/10.1016/j.jallcom.2019.05.038.
Wei S, Di Lecce D, Messini D’Agostini R, Hassoun J. Synthesis of a high-capacity α-FE2O3@c conversion anode and a high-voltage lini0.5mn1.5O4 spinel cathode and their combination in a Li-Ion Battery. ACS Appl Energy Mater. 2021;4(8),8340–8349. https://doi.org/10.1021/acsaem.1c01585.
Lim AS, Kim J, Hwa Y, Cho KY, Yoon S. FE2O3/n-doped carbon-modified SiOx particles via Ionic liquid as anode materials for Li-ion batteries. J Appl Electrochem. 2022;52(8):1163–71. https://doi.org/10.1007/s10800-022-01700-2.
Duan J, Ji K, Min L, Zhang Y, Yang T, Chen M, Wang C. Hybrid electrolyte-mediated nano-scaled γ-FE2O3 cathode for emerging aqueous zinc battery. Electrochim Acta. 2021;390: 138883. https://doi.org/10.1016/j.electacta.2021.138883.
Feng Y, Shu N, Xie J, Ke F, Zhu Y, Zhu J. Carbon-coated fe2o3 hollow sea urchin nanostructures as high-performance anode materials for lithium-ion battery. Sci China Mater. 2020;64(2):307–17. https://doi.org/10.1007/s40843-020-1437-2.
Li Z, Mao Y, Tian Q, Zhang W, Yang L. Extremely facile preparation of high-performance Fe2O3 anode for lithium-ion batteries. J Alloy Compd. 2019;784:125–33. https://doi.org/10.1016/j.jallcom.2018.12.392.
Jiang Y, Zhang D, Li Y, Yuan T, Bahlawane N, Liang C, Sun W, Lu Y. Material for lithium ion batteries. Nano Energy. 2013;4:23–30. https://doi.org/10.1016/j.nanoen.2013.12.001.
Liu X, Si W, Zhang J, Sun X, Deng J, Baunack S, Oswald S, Liu L, Yan C, Schmidt OG. Free-standing FE2O3 nanomembranes enabling ultra-long cycling life and high rate capability for Li-Ion Batteries. Scientific Reports. 2014; 4(1). https://doi.org/10.1038/srep07452.
Wang C, Sireesha P, Shang J, McCloy JS, Liu J, Zhong W-H. Converting commercial FE2O3 to effective anode material using glucose as “etching” agent. Ceram Int. 2023;49(20):32652–62. https://doi.org/10.1016/j.ceramint.2023.07.234.
Sarradin J. Study of FE2O3-based thin film electrodes for lithium-ion batteries. Solid State Ionics. 1998;112(1–2):35–40. https://doi.org/10.1016/s0167-2738(98)00211-2.
Needham SA, Wang GX, Konstantinov K, Tournayre Y, Lao Z, Liu HK. Electrochemical performance of co[sub 3]o[sub 4]–C composite anode materials. Electrochem Solid-State Lett. 2016;9(7). https://doi.org/10.1149/1.2197108.
Reddy MV, Yu T, Sow CH, Shen ZX, Lim CT, Subba Rao GV, Chowdari BVR. Α-FE2O3 nanoflakes as an anode material for Li-ion batteries. Adv Func Mater. 2007;17(15):2792–9. https://doi.org/10.1002/adfm.200601186.
Chen JS, Zhu T, Yang XH, Yang HG, Lou XW. Top-down fabrication of α-FE2O3 single-crystal nanodiscs and microparticles with tunable porosity for largely improved lithium storage properties. J Am Chem Soc. 2010;132(38):13162–4. https://doi.org/10.1021/ja1060438.
Xu S, Hessel CM, Ren H, Yu R, Jin Q, Yang M, Zhao H, Wang D. Α-FE2O3multi-shelled hollow microspheres for lithium ion battery anodes with superior capacity and charge retention. Energy Environ Sci. 2014;7(2):632–7. https://doi.org/10.1039/c3ee43319f.
Wang B, Wang G, Wang H. Synthesis and electrochemical investigation of hollow hierarchical metal oxide microspheres for high performance lithium-ion batteries. Electrochim Acta. 2015;156:1–10. https://doi.org/10.1016/j.electacta.2014.10.157.
Zhang J, Huang T, Liu Z, Yu A. Mesoporous Fe2O3 nanoparticles as high performance anode materials for lithium-ion batteries. Electrochem Commun. 2013;29:17–20. https://doi.org/10.1016/j.elecom.2013.01.002.
Chen L, Xu H, Li L, Wu F, Yang J, Qian Y. A comparative study of lithium-storage performances of hematite: Nanotubes vs. Nanorods J Power Sources. 2014;245:429–35. https://doi.org/10.1016/j.jpowsour.2013.06.154.
Cherian CT, Sundaramurthy J, Kalaivani M, Ragupathy P, Kumar PS, Thavasi V, Reddy MV, Sow CH, Mhaisalkar SG, Ramakrishna S, Chowdari BV. Electrospun α-Fe2O3 nanorods as a stable, high capacity anode material for Li-Ion Batteries. J Mater Chem. 2012;22(24):12198. https://doi.org/10.1039/c2jm31053h.
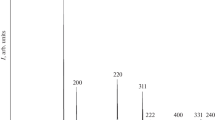
Takai S, Sawada E, Harada J, Park S, Oda M, Esaki S, Nishijima M, Yoshie T, Yabutsuka T, Yao T. Synthesis and anode properties of corundum-type structured (Fe2O3) 1-X (Al2O3) x solid solutions in the whole compositional range. Solid State Ionics. 2017;313:1–6. https://doi.org/10.1016/j.ssi.2017.10.026.
Powder diffraction file: Sets 21 to 22: Organic Volume No. pdis-22orb /compiled by JCPDS, in cooperation with the American Society for Testing and Materials, the Institute of Physics, (1980), JCPDS.
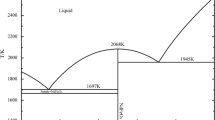
Muan A. On the stability of the phase fe 2 O 3 .al 2 O 3. Am J Sci. 1958;256(6), 413–422. https://doi.org/10.2475/ajs.256.6.413.
Muan A, Gee CL. Phase equilibrium studies in the system iron oxide-Al2O3 in air and at 1 atm. O2 pressure. J. Am. Ceramic Soc. 1956;39(6), 207–214. https://doi.org/10.1111/j.1151-2916.1956.tb15647.x.
Polli AD, Lange FF, Levi CG, Mayer J. Crystallization behavior and microstructure evolution of (Al, Fe)2O3 synthesized from liquid precursors. J Am Ceram Soc. 1996;79(7):1745–55. https://doi.org/10.1111/j.1151-2916.1996.tb07991.x.
Nakaishi H, Yabutsuka T, Yao T, Kitao S, Seto M, Chen W, Shimonishi Y, Yoshida S, Takai S. Homogeneous solid-solution formation in Fe2O3–Al2O3 system observed by TEM, XAFS, and Mössbauer spectroscopy. Mater Chem Phys. 2023;303: 127764. https://doi.org/10.1016/j.matchemphys.2023.127764.
Rosen PF, Woodfield BF. Standard methods for heat capacity measurements on a quantum design physical property measurement system. J Chem Thermodyn. 2020;141: 105974. https://doi.org/10.1016/j.jct.2019.105974.
Lashley JC, Hundley MF, Migliori A, Sarrao JL, Pagliuso PG, Darling TW, Jaime M, Cooley JC, Hults WL, Morales L, Thoma DJ, Smith JL, Boerio-Goates J, Woodfield BF, Stewart GR, Fisher RA, Phillips NE. Critical examination of heat capacity measurements made on a quantum design physical property measurement system. Cryogenics. 2003;43(6):369–78. https://doi.org/10.1016/s0011-2275(03)00092-4.
Chase MW. NIST-JANAF Thermochemical Tables, 1988.
Roy A, Sreeramagiri P, Babuska T, Krick B, Ray PK, Balasubramanian G. Lattice distortion as an estimator of solid solution strengthening in high-entropy alloys. Mater Charact. 2021;172: 110877. https://doi.org/10.1016/j.matchar.2021.110877.
Petric A, Jacob KT, Alcock CB. Thermodynamic properties of Fe3O4-FeAl2O4 spinel solid solutions. J Am Ceram Soc. 1981;64(11):632–9. https://doi.org/10.1111/j.1151-2916.1981.tb15860.x.
Tian F, Yang Z, Lin D-Y, Zhao Y-F. Lattice distortion inducing local antiferromagnetic behaviors in feal alloys. J Phys: Condens Matter. 2020;32(46): 465805. https://doi.org/10.1088/1361-648x/abae1b.
Fultz B. Vibrational thermodynamics of materials. Prog Mater Sci. 2010;55(4):247–352. https://doi.org/10.1016/j.pmatsci.2009.05.002.
Gao MC, Zhang C, Gao P, Zhang F, Ouyang LZ, Widom M, Hawk JA. Thermodynamics of concentrated solid solution alloys. Curr Opin Solid State Mater Sci. 2017;21(5):238–51. https://doi.org/10.1016/j.cossms.2017.08.001.
Takai S, Nakanishi T, Oikawa K, Torii S, Hoshikawa A, Kamiyama T, Esaka T.. Neutron diffraction and IR spectroscopy on mechanically alloyed La-substituted PbWO4. Solid State Ionics. 2004;170(3–4), 297–304. https://doi.org/10.1016/j.ssi.2004.03.008.
Takai S, Nakanishi T, Tojo T, et al. Low temperature heat capacities of mechanically alloyed La-doped PbWO4 system. J Therm Anal Calorim. 2002;69:805–11. https://doi.org/10.1023/A:1020695620191.
Galsin JS. Defects in crystalline solids. Solid State Physics. 2019; 513–537. https://doi.org/10.1016/b978-0-12-817103-5.00023-2.
Navrotsky A. Physics and Chemistry of Earth Materials. Cambridge University Press, Cambridgee; 1994. https://doi.org/10.1017/CBO9781139173650.007.
Wang Z, Fang Q, Li J, Liu B, Liu Y. Effect of lattice distortion on solid solution strengthening of BCC high-entropy alloys. J Mater Sci Technol. 2018;34(2):349–54. https://doi.org/10.1016/j.jmst.2017.07.013.
Kim JH, Hyunyong C, Back SY, Yun J, Lee HS, Rhyee J. Lattice distortion and anisotropic thermoelectric properties in hot-deformed CuI-doped Bi2Te2·7Se0.3. J Alloys Compounds. 2020;815, 152649. https://doi.org/10.1016/j.jallcom.2019.152649.
Zhang F, Yang T, Jin K, Bei H, Weber WJ, Zhang Y. Lattice distortion and phase stability of PD-Doped NICOFECR Solid-Solution alloys. Entropy. 2018;20(12):900. https://doi.org/10.3390/e20120900.
Hartmann WM, Culbert HV, Huebener RP. Enhancement of the lattice heat capacity due to Low-Frequency resonance modes in dilute Aluminum-Silver alloys. Phys Rev. 1970;1(4):1486–93. https://doi.org/10.1103/physrevb.1.1486.
Ziemniak S, Anovitz LM, Castelli RA, Porter WD. Magnetic contribution to heat capacity and entropy of nickel ferrite (NiFe2O4). J Phys Chem Solids. 2007;68(1):10–21. https://doi.org/10.1016/j.jpcs.2006.07.015.
Eгopышeвa AB, Эллepт O, Popova EF, Tyurin AV, Xopoшилoв A, Kirdyankin DI, Гaвpичeв КC. Heat capacity, thermodynamic and magnetic properties of the pyrochlore-like compounds RE2FeTaO7. J Chem Thermodyn. 2021;161: 106565. https://doi.org/10.1016/j.jct.2021.106565.
Lin J, Wu TB. Effects of isovalent substitutions on lattice softening and transition character of BaTiO3 solid solutions. J Appl Phys. 1990;68(3):985–93. https://doi.org/10.1063/1.346665.
Zhang S, Zhang HB, Zhao F, Jiang M, Xiao H, Liu Z, Zu X. Impact of isovalent and aliovalent substitution on the mechanical and thermal properties of Gd2Zr2O7. Scientific Reports. 2017;7(1). https://doi.org/10.1038/s41598-017-06725-8.
Guskov AV, Гaгapин ПГ, Guskov VN, Tyurin AV, Гaвpичeв КC. Thermodynamic functions of DY2O3⋅2HFO2 solid solution and the Schottky anomaly. Russ J Phys Chem A. 2022;96(9):1831–9. https://doi.org/10.1134/s003602442209014x.
Kobashi K, Oguni M. Calorimetric finding of an anomaly of a schottky type peculiar to solid solutions in a chlorocyclohexane-bromocyclohexane system. J Phys Chem Solids. 1997;58(5):695–9. https://doi.org/10.1016/s0022-3697(97)00206-0.
Mitroshenkov NV, Matovnikov AV, Кyзнeцoв CB, Lazutkina M, Volchek A, Konoplin NA, Kornev BI, Hoвикoв BB. Low-temperature anomalies of thermodynamic properties of lanthanum trifluoride LaF3 and (SrF2)0.5 (LaF3)0.5 multivalent solid solution. Journal of Alloys and Compounds. 2022;894, 162537. https://doi.org/10.1016/j.jallcom.2021.162537.
Babichuk IS, Romaniuk YA, Golovynskyi S, Hurtavy VG, Myдpый AB, Zhivulko VD, Babichuk I, Xu C, Lin C, Cao M, Hreshchuk O, Yukhymchuk V, Valakh MY, Li B, Yang J. Spectroscopy and theoretical modeling of phonon vibration modes and band gap energy of Cu2ZnSn(SxSe1–x)4 bulk crystals and thin films. ACS Omega. 2021;6(43):29137–48. https://doi.org/10.1021/acsomega.1c04356.
Eifler A, Riede V, Brückner J, Weise S, Krāmer V, Lippold G, Schmitz W, Bente K, Grill W. 1Band gap energies and lattice vibrations of the lithium ternary compounds LiInSe2, LiInS2, LiGaSe2 and LiGaS2. Jpn J Appl Phys. 2000;39(S1):279. https://doi.org/10.7567/jjaps.39s1.279.
Лeбeдeв AИ, Sluchinskaya IA. Low-temperature phase transitions in some quaternary solid solutions of IV-VI semiconductors. J Alloy Compd. 1994;203:51–4. https://doi.org/10.1016/0925-8388(94)90713-7.
Pal-Val PP, Pal-Val L, Ostapovets A, Vaněk P. Low temperature kinetics of In-CD solid solution decomposition. Solid State Phenom. 2008;137:35–42. https://doi.org/10.4028/www.scientific.net/ssp.137.35.
Acknowledgements
This work was partly supported by the Collaborative Research Projects of Laboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology.
Author information
Authors and Affiliations
Contributions
D. Luo analyzed the data and drafted the manuscript. T. Yabutsuka supported the experiments. T. Yao conceived the solid solution formation through Mechanochemical Alloying. S. Kitani and H. Kawaji performed the heat capacity measurements. S. Takai organized the study and refined the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, D., Yabutsuka, T., Yao, T. et al. Low-temperature heat-capacities of corundum-type structured (Fe2O3)1−x(Al2O3)x solid solutions with x = 0.25, 0.50 and 0.75. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13231-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13231-3