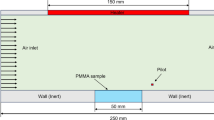
Abstract
In the dispersing process of liquid fuel or metal powder under the explosion load of a high explosive, the explosion product of central high-energy explosive expands. Flow of the explosion product with high temperature is coupled with the dispersing of fuel driven by the explosion load. When concentration of the dispersing fuel cloud and the current temperature meet critical condition for igniting the cloud, the fuel cloud will be ignited. If the dispersing fuel is ignited by the high temperature of the explosion product, the fuel cloud will burn, and the pre-designed detonation energy output cannot be achieved. Therefore, avoiding the ignition phenomenon in the process of fuel dispersion is an important issue in effective utilization of multi-phase cloud explosion energy. The minimum ignition temperature (MIT) of gas–liquid two-phase cloud is the basis for avoiding the dispersing fuel to be ignited under explosion load of a high explosive. In this study, the MITs of gas–liquid two-phase cloud of ethanol, octane, nitromethane, and isopropyl nitrate were measured. The MIT of gas–liquid two-phase cloud is a function of ignition temperature (IT) of vapor and atomization parameters of liquid fuel. The MIT of gas–liquid two-phase cloud of a liquid fuel is higher than IT of vapor of the liquid fuel. The MIT of gas–liquid two-phase cloud decreases with the decrease in droplet characteristic sizes. The prediction model of MIT of gas–liquid two-phase cloud of liquid fuel (MIT = 507 + 0.15 \(\beta^{0.8} T_{{\text{i}}}\)) has been established in this study. If the ignition temperature of vapor of a liquid fuel and the atomization characteristic parameters of the liquid fuel are known, the MIT of gas–liquid two-phase cloud of the liquid fuel cloud be determined. The measured MITs of gas–liquid two-phase cloud for octane (C8H18), ethanol (C2H6O), nitromethane (CH3NO2), and isopropyl nitrate (C3H7NO3) are 616 K, 737 K, 692 K, and 671 K, respectively. The MITs of gas–liquid two-phase cloud predicted using the model in this study for the four liquid fuels are 621 K, 740 K, 690 K, and 663 K, respectively. The relative error of the predicted MITs is less than 3.5%.















Similar content being viewed by others
References
Apparao A, Saji J, Balaji M, Devangan AK, Rao CR. Determination of minimum mass and spatial location of initiator for detonation of propylene oxide aerosols. Shock Waves. 2017;2017(27):247–255s.
Li YH, Song ZD, Li YZ, Lang Y, Li D. Theoretical analysis and numerical simulation for the spill procedure of liquid fuel of fuel air explosive with shell. Int J Nonlin Mech. 2010;45(7):699–703.
Ye CL, Du QL, Zhang Q, Liu LJ. Flame behavior, shock wave, and instantaneous thermal field generated by unconfined vapor-liquid propylene oxide/air cloud detonation. Def Technol. 2022. https://doi.org/10.1016/j.dt.2022.05.002.
Strizhak P, Volkov R, Moussa O, Tarlet D, Bellettre J. Child droplets from micro-explosion of emulsion and immiscible two-component droplets. Int J Heat Mass Tran. 2021. https://doi.org/10.1016/j.ijheatmasstransfer.2021.120931.
Fox M, Hastings R, Lovald S, Heinrich J. Case study of an aerosol explosion and a method to determine explosion temperatures. J Fail Anal Prev. 2007;7:165–74.
Song X, Zhang J, Zhang D, Xie L, Li B. Dispersion and explosion characteristics of unconfined detonable aerosol and its consequence analysis to humans and buildings. Process Saf Environ Prot. 2021;152:66–82.
Kubo M, Nakaoka A, Morimoto K, Shimada M, Horie M, Morimoto Y, Sasaki T. Aerosol generation by a spray-drying technique under coulomb explosion and rapid evaporation for the preparation of aerosol particles for inhalation tests. Aerosol Sci Tech. 2014;48:698–705.
Pei B, Yu M, Chen L, Wang F, Yang Y, Zhu X. Experimental study on the synergistic inhibition effect of gas-liquid two phase medium on gas explosion. J Loss Prevent Process Ind. 2017;49:797–804.
Preiss FJ, Rütten E, Tröster A, Gräf, Karbstein H. Influence of the droplet trajectory on the resulting droplet deformation and droplet size distribution in high-pressure homogenizer orifices. Can J Chem Eng. 2022;100:1451–67.
Rostampour A, Shojaeefard MH, Molaeimanesh GR. Role of water micro-explosion on fuel droplet size distribution, engine performance, and emissions in a water-diesel emulsified engine: a comprehensive numerical investigation. Int J Engine Res. 2022. https://doi.org/10.1177/14680874221076445.
Rosli MAF, Aziz ARA, Smael MA, Elbashir INO, Zainal A, Ezrann Z, Baharom M, Mohammed SE. Experimental study of micro-explosion and puffing of gas-to-liquid (GTL) fuel blends by suspended droplet method. Energy. 2020. https://doi.org/10.1016/j.energy.2020.119462.
Fdida N, Vingert L, Ristori A, Le SY. Droplet size and velocity measurements in a cryogenic jet flame of a rocket-type combustor using high-speed imaging. Atomization Spray. 2016;26:411–38.
Ferrão S, Inês A, Silva ARR, Moita ASOH, Mendes MAA, Costa MMG. Combustion characteristics of a single droplet of hydroprocessed vegetable oil blended with aluminum nanoparticles in a drop tube furnace. Fuel. 2021. https://doi.org/10.1016/j.fuel.2021.121160.
Zhou ZF, Yin J, Yang XY, Chen B, Liu B. Experimental investigation on the macroscopic spray and microscopic droplet diameter, velocity and temperature of R404A flashing spray. Int J Heat Mass Tran. 2021. https://doi.org/10.1016/j.ijheatmasstransfer.2021.121546.
Li H, Rosebrock CD, Wu Y, Wriedt T, Mädler L. Single droplet combustion of precursor/solvent solutions for nanoparticle production: optical diagnostics on single isolated burning droplets with micro-explosions. P Combust Inst. 2019;37:1203–11.
Yuan S, Ji C, Monhollen A, Kwon JS, Mashuga C. Experimental and thermodynamic study of aerosol explosions in a 36 L apparatus. Fuel. 2019;245:467–77.
Arshad U, Taqvi SAA, Buang A. Modelling of the minimum ignition temperature (MIT) of corn dust using statistical analysis and artificial neural networks based on the synergistic effect of concentration and dispersion pressure. Process Saf Environ. 2021;147:742–55.
Deng J, Qu J, Wang Q, Zhai X, Xiao Y, Cheng Y, Shu C. Minimum ignition temperature of aluminium dust clouds via the Godbert-Greenwald furnace. Process Saf Environ. 2019;129:176–83.
Mishra DP, Azam S. Experimental investigation on effects of particle size, dust concentration and dust-dispersion-air pressure on minimum ignition temperature and combustion process of coal dust clouds in a G-G furnace. Fuel. 2018;227:424–33.
Wu D, Norman F, Verplaetsen F, Van den Bulck E. Experimental study on the minimum ignition temperature of coal dust clouds in oxy-fuel combustion atmospheres. J Hazard Mater. 2016;307:274–80.
Addai EK, Gabel D, Krause U. Experimental investigations of the minimum ignition energy and the minimum ignition temperature of inert and combustible dust cloud mixtures. J Hazard Mater. 2016;307:302–11.
Addai EK, Gabel D, Krause U. Models to estimate the minimum ignition temperature of dusts and hybrid mixtures. J Hazard Mater. 2016;304:73–83.
Chen T, Van Caneghem J, Degrève J, Berghmans J, Verplaetsen F, Vanierschot M. Comparison between a numerical model and the classic thermal explosion theories for the calculation of the sminimum ignition temperature of dust clouds. Process Saf Environ. 2022;166:222–31.
Liu X, Wang Y, Zhang Q. A study of the explosion parameters of vapor–liquid two-phase JP-10/air mixtures. Fuel. 2016;165:279–88.
Jia Y, Zhang C, Zhang Q, Liang H. Correlation between concentration, droplet size, turbulent intensity and explosion parameters of gas-liquid two-phase mixture. Fire Saf J. 2023;136: 103731.
Liu X, Zhang Q, Wang Y. Influence of particle size on the explosion parameters in two-phase vapor–liquid n-hexane/air mixtures. Process Saf Environ. 2015;95:184–94.
Kuo KK. Principles of combustion. 2nd ed. Hoboken: Wiley; 2005.
Zhou Z, Kar T, Yang Y, Brear M, Leone TG, Anderson JE, Shelby MH, Curtis E, Lacey J. The significance of octane numbers to drive cycle fuel efficiency. Fuel. 2021;302: 121095.
Shi H, Tang Q, Uddeen K, Johansson B, Turner J, Magnotti G. Effects of multiple spark ignition on engine knock under different compression ratio and fuel octane number conditions. Fuel. 2022;310:122471.
Lopez-Pintor D, Dec JE. Experimental evaluation of a gasoline-like fuel blend with high renewable content to simultaneously increase–sensitivity, RON, and octane sensitivity. Energ Fuel. 2021;35(20):16482–93.
Zhang C, Bai C, Ren J, Chang C, Yao J. The promotion of nitromethane on solid–liquid fuel/air mixtures explosion characteristics under different ambient conditions. Fuel. 2022;322: 124190.
Bayar CC. Investigation of the radical nitration of isooctane fuel via nitromethane propellant. Cent Eur J Energ Mat. 2018;15(2):225–42.
Gao Z, Yang M, Tang C, Yang F, Yang K, Deng F, Huang Z. Measurements of the high temperature ignition delay times and kinetic modeling study on oxidation of nitromethane. Combust Sci Technol. 2020;192(2):313–34.
Yang M, Gao Z, Tang C, Xu Z, Gao Z, Hu E, Huang Z. Nitromethane pyrolysis and oxidation in a jet-stirred reactor: experimental measurements, kinetic model validation and interpretation. Fuel. 2020;263: 116491.
Hou Q, Liu W, Chu Z, et al. Exploring the chemistry behind low temperature auto-ignition of isopropyl nitrate in an RCM: an experimental and kinetic modeling study. P Combust Inst. 2022. https://doi.org/10.1016/j.proci.2022.10.022.
Cao J. Atomics. Beijing: China Machine Press; 2005.
Acknowledgements
The research presented in this paper was supported by State Key Laboratory of Precision Blasting and Hubei Key Laboratory of Blasting Engineering, Jianghan University (No. PBSKL2022A02). Thanks to Mr. Cuyan Sun and Mr. Hang Sun for participating in part of the experiment.
Author information
Authors and Affiliations
Contributions
YJ contributed to writing—original draft. QZ helped in conceptualization and methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, Y., Zhang, Q. Minimum ignition temperature of gas–liquid two-phase cloud. J Therm Anal Calorim 149, 3819–3831 (2024). https://doi.org/10.1007/s10973-024-12990-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-024-12990-3