Abstract
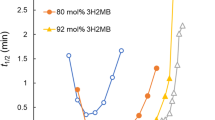
A linear diamides derivative (TMC-300) was incorporated into biosourced and biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P34HB) to investigate the influence of TMC-300 on the crystallization and rheological properties of P34HB. TMC-300 displayed an outstanding nucleating effect on P34HB. Adding 0.5 mass% TMC-300 increased the non-isothermal crystallization temperature by about 20 °C at rate of 5 °C min−1. Isothermal crystallization half-time at 95 °C obviously decreased from 20 min for neat P34HB to 2.5 min for P34HB/0.2TMC. Adding TMC-300 dramatically increased nucleation density and reduced spherulite size. Moreover, the rheological properties were obviously enhanced by adding TMC-300. The most intriguing result was that percolation network was formed at TMC-300 content between 0.5 and 1 mass%, which led to the transition of melt behaviors from liquid-like to solid-like. The unusual combination of nucleating effect and rheological properties promotion of TMC-300 on P34HB was in great potential for expanding practical application of biosourced and biodegradable P34HB.












Similar content being viewed by others
References
Grząbka-Zasadzińska A, Odalanowska M, Borysiak S. Thermal and mechanical properties of biodegradable composites with nanometric cellulose. J Therm Anal Calorim. 2019;138:4407–16.
Kunwar B, Cheng HN, Chandrashekaran SR, Sharma BK. Plastics to fuel: a review. Renew Sust Energy Rev. 2016;54:421–8.
Zhou X, Wu T, Wang X. Preparation and properties of biodegradable multi-block copolymer/graphite oxide composite phase change materials. J Therm Anal Calorim. 2021;143:3401–8.
Fenni SE, Wang J, Haddaoui N, Favis BD, Müller AJ, Cavallo D. Crystallization and self-nucleation of PLA, PBS and PCL in their immiscible binary and ternary blends. Thermochim Acta. 2019;677:117–30.
Jia K, Cao R, Hua G, Li P. Study of class I and class III polyhydroxyalkanoate (PHA) synthases with substrates containing a modified side chain. Biomacromolecules. 2016;17:1477–85.
Che X, Ye H, Chen G. Effects of uracil on crystallization and rheological property of poly(R-3-hydroxybutyrate-co-4-hydroxybutyrate). Compos Part A Appl Sci. 2018;109:141–50.
Barham PJ, Keller A, Otun EL, Holmes EL. Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. J Mater Sci. 1984;19:2781–94.
Mangeon C, Michely L, Rios De Anda A, Thevenieau F, Renard E, Langlois V. Natural terpenes used as plasticizers for poly(3-hydroxybutyrate). ACS Sustain Chem Eng. 2018;6:16160–8.
Larsson M, Hetherington CJD, Wallenberg R, Jannasch P. Effect of hydrophobically modified graphene oxide on the properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polymer. 2017;108:66–77.
Wee CY, Liow SS, Li Z, Wu Y, Loh XJ. New poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate] (P3HB4HB)-based thermogels. Macromol Chem Phys. 2017;218:1700196.
Wang L, Wang X, Zhu W, Chen Z, Pan J, Xu K. Effect of nucleation agents on the crystallization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate)(P3/4HB). J Appl Polym Sci. 2010;116:1116–23.
Xia MQ, Zhang YF. The relation between chemical structure of branched amide nucleating agents and nucleation effect in isotactic polypropylene. J Therm Anal Calorim. 2021;145:3053–66.
Pan P, Liang Z, Nakamura N, Miyagawa T, Inoue Y. Uracil as nucleating agent for bacterial poly[(3-Hydroxybutyrate)-co-(3-hydroxyhexanoate)] copolymers. Macromol Biosci. 2009;9:585–95.
Vandewijngaarden J, Murariu M, Dubois P, Carleer R, Yperman J, D’Haen J, Peeters R, Buntinx M. Effect of ultrafine talc on crystallization and end-use properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J Appl Polym Sci. 2016;133:43808.
Kai W, He Y, Inoue Y. Fast crystallization of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with talc and boron nitride as nucleating agents. Polym Int. 2005;54:780–9.
Xu C, Qiu Z. Crystallization behavior and thermal property of biodegradable poly(3-hydroxybutyrate)/multi-walled carbon nanotubes nanocomposite. Polym Adv Technol. 2011;22:538–44.
Miao Y, Fang C, Shi D, Li Y, Wang Z. Coupling effects of boron nitride and heat treatment on crystallization, mechanical properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). Polymer. 2022;252:124967.
Jing X, Qiu Q. Crystallization kinetics and thermal property of biodegradable poly(3-hydroxybutyrate)/graphene oxide nanocomposites. J Nanosci Nanotechnol. 2012;12:7314–21.
El-Hadi AM. Investigation of the effect of nano-clay type on the non-isothermal crystallization kinetics and morphology of poly(3(R)-hydroxybutyrate) PHB/clay nanocomposites. Polym Bull. 2014;71:1449–70.
Xu PW, Cao Y, Lv P, Ma PM, Dong WF, Bai HY, Wang W, Du ML, Chen M. Enhanced crystallization kinetics of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyhexanate) with structural optimization of oxalamide compounds as nucleators. Polym Degrad Stab. 2018;154:170–6.
Chen J, Tong W, Xu C, Wu D, Pan K. Insights into the nucleation role of cellulose crystals during crystallization of poly(β-hydroxybutyrate). Carbohydr Polym. 2015;134:508–15.
Dong T, Mori T, Aoyama T, Inoue Y. Rapid crystallization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer accelerated by cyclodextrin-complex as nucleating agent. Carbohydr Polym. 2010;80:387–93.
El-Taweel SH. Effect of benzoic acid on the crystallization behavior of poly(3-hydroxybutyrate). J Macromol Sci Part B. 2013;52:1521–30.
Zhang Y, Zhou P, Mao J, Liu N. Influences of octamethylenedicarboxylic dibenzoylhydrazide on crystallization, melting behaviors, and properties of isotactic polypropylene. Polym Bull. 2019;76:1685–96.
Zhou P, Zhang Y, Lin X. Crystallization kinetics of isotactic polypropylene nucleated with octamethylenedicarboxylic dibenzoylhydrazide under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2019;136:749–57.
Zhan Y, Mao J. Effect of chemical structure of hydrazide compounds on nucleation effect in isotactic polypropylene. J Polym Res. 2019;26:277.
Liu S, He Y, Qu J. Manufacturing high-performance polylactide by constructing 3D network crystalline structure with adding self-assembly nucleator. Ind Eng Chem Res. 2022;61:4567–78.
Li L, Yang L, Tang J, Yang J, Li W, Zhou S, Ma H, Zhu H, Zhu Z. Modulated crystallization behavior of bacterial copolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate): effect of a linear multiple amides derivative as a nucleator. J Macromol Sci A. 2020;57:439–50.
Tang Y, Wang Y, Chen S, Wang X. Fabrication of low-density poly(lactic acid) microcellular foam by self-assembly crystallization nucleating agent. Polym Degrad Stab. 2022;198:109891.
Jiang X, Luo S, Sun K, Chen X. Effect of nucleating agents on crystallization kinetics of PET. Express Polym Lett. 2007;4:245–51.
Zhou S, Sun Y, Ma H, Jia C, Sun X, Yang Y, Liu J, Yang J. Linear diamides derivative-nucleated biodegradable poly(ethylene succinate) polyester: crystallization kinetics and aggregated structure manipulated by hydrogen bond interaction. J Polym Environ. 2021;29:3605–17.
Bai H, Zhang W, Deng H, Zhang Q, Fu Q. Control of crystal morphology in poly(L-lactide) by adding nucleating agent. Macromolecules. 2011;44:1233–7.
Luo R, Xu K, Chen G. Study of miscibility, crystallization, mechanical properties, and thermal stability of blends of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J Appl Polym Sci. 2007;105:3402–8.
Li J, Qiu Z. Effect of low loadings of cellulose nanocrystals on the significantly enhanced crystallization of biodegradable poly(butylene succinate-co-butylene adipate). Carbohydr Polym. 2019;205:211–6.
Avrami M. Kinetics of phase change. II transformation: time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24.
Avrami M. Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941;9:177–84.
Lorenzo AT, Arnal ML, Albuerne J, Müller AJ. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: guidelines to avoid common problems. Polym Test. 2007;26:222–31.
Qiu Z, Li Z. Effect of orotic acid on the crystallization kinetics and morphology of biodegradable poly(L-lactide) as an efficient nucleating agent. Ind Eng Chem Res. 2011;50:12299–303.
Dong T, Kai W, Pan P, Cao A, Inoue Y. Effects of host guest stoichiometry of a-cyclodextrin aliphatic polyester inclusion complexes and molecular weight of guest polymer on the crystallization behavior of aliphatic polyesters. Macromolecules. 2007;40:7244–51.
Pan H, Qiu Q. Biodegradable poly(L-lactide)/polyhedral oligomeric silsesquioxanes nanocomposites: enhanced crystallization, mechanical properties, and hydrolytic degradation. Macromolecules. 2010;43:1499–506.
Li Y, Huang H, Wang Z, He T. Tuning radial lamellar packing and orientation into diverse ring-banded spherulites: effects of structural feature and crystallization condition. Macromolecules. 2014;47:1783–92.
Kunioka M, Tamaki A, Doi Y. Crystalline and thermal properties of bacterial copolyesters: poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules. 1989;22:694–7.
Zhao H, Bian Y, Li Y, Dong Q, Han C, Dong L. Bioresource-based blends of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and stereocomplex polylactide with improved rheological and mechanical properties and enzymatic hydrolysis. J Mater Chem A. 2014;2:8881–92.
Wen X, Lu X, Peng Q, Zhu F, Zheng N. Crystallization behaviors and morphology of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J Therm Anal Calorim. 2012;109:1–8.
Arrigo R, Malucelli G. Rheological behavior of polymer/carbon nanotube composites: an overview. Materials. 2020;13:2771.
Han CD, Kim JK. On the use of time-temperature superposition in multicomponent/multiphase polymer systems. Polymer. 1993;34:2533–9.
Han CD, Kim J. Rheological technique for determining the order-disorder transition of block copolymers. J Polym Sci B Polym Phys. 1987;25:1741–64.
Acknowledgements
This work is supported by the Science and Technology Research Project of Education Department of Jilin Province (JJKH20230325KJ).
Author information
Authors and Affiliations
Contributions
YL contributed to data curation, writing—original draft, investigation, visualization, resources, project administration, methodology, and conceptualization. CH contributed to data curation, investigation, writing—review and editing, and visualization. DL contributed to data curation, formal analysis, supervision, and conceptualization. HC contributed to software and data curation. LX contributed to formal analysis and conceptualization. BW contributed to data curation and formal analysis.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Han, C., Li, D. et al. Effects of linear diamides derivative nucleating agent on the enhanced crystallization and rheological properties of biosourced and biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J Therm Anal Calorim 149, 1003–1014 (2024). https://doi.org/10.1007/s10973-023-12790-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12790-1