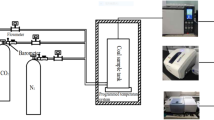
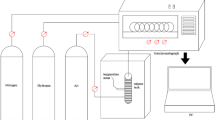
Abstract
Aimed at the deprived research on physicochemical synergistic inhibition and the micromechanism of inhibition. The inhibitory effect and mechanism of MgCl2, TPPI and the combination of them were studied in this research. Low-temperature oxidation gas production experiment and synchronous thermal analyzer were used to analyze the inhibition and thermal behavior effects of different inhibitors, respectively. Fourier transform infrared spectrometer experiment was used to analyze the changes of the surface functional groups on coal samples before and after inhibition. Density functional theory was used to reveal the inhibitory mechanism of TPPI. In the low-temperature oxidation experiment, the CO release of Composite Inhibitor 5 (TPPI:MgCl2 = 1:1) treatment coal samples was the least. From the characteristic temperature points and heat release, it can be seen that the composite inhibitor reflects the physicochemical synergistic effect. The addition of MgCl2 will enhance the inhibitory effect at low-temperature stage (< 400°C) and weaken the inhibitory effect at high-temperature stage (> 400°C). Composite Inhibitor 3 (TPPI:MgCl2 = 3:7) showed strong inhibitory effect in both low and high-temperature phases, the comprehensive comparison showed the best inhibitory effect. The contents of aliphatic functional groups, hydroxyl groups and carboxyl groups decreased in the coal samples treated by TPPI and composite inhibitor, while the relative contents of stable ether bond and aromatic functional groups increased. The inhibitory mechanism of TPPI is to remove the free radical as R–COO·, R–CO· and R–C· by using itself and its hydrolytic products, to interrupt the chain reaction and finally to inhibit coal spontaneous combustion. The conclusions of this article provide theoretical support for the preparation of physicochemical synergistic inhibitors and the revelation of microscopic mechanism of TPPI inhibition on coal spontaneous combustion.



















Similar content being viewed by others
References
BP. BP Statistical Review of World Energy 2022 2022. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022.
Di Gianfrancesco A. Worldwide overview and trend for clean and efficient use of coal. Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants, 2017, pp 643–687
Li QW, Xiao Y, Zhong KQ, Shu CM, Lü HF, Deng J, et al. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel. 2020;275:117981.
Zhu HQ, Huo YJ, Wang W, He X, Fang SH, Zhang YL. Quantum chemical calculation of reaction characteristics of hydroxyl at different positions during coal spontaneous combustion. Process Saf Environ Prot 148:624–635.
Zhu HQ, Qu BL, Liao Q, Xie LH, Wang JX, Hu LT et al. Evolution and mechanism for the terahertz dielectric spectrum of coal during oxidation. Infrared Phys. Technol.2022;127.
Pan RK, Ma JW, Fu D, Li C, Jia HL, Zheng LG. Experimental study on the new environmental protection chemical composite inhibitor for the inhibition of coal spontaneous combustion. J Therm Anal Calorim. 2020;139:37–45.
Tang Y. Experimental investigation of applying MgCl2 and phosphates to synergistically inhibit the spontaneous combustion of coal. J Energy Inst. 2018;91:639–45.
Shi T, Wang XF, Deng J, Wen ZY. The mechanism at the initial stage of the room-temperature oxidation of coal. Combust Flame. 2005;140(4):332–45.
Qi XY, Wang DM, Xin HH, Qi GS. An in situ testing method for analyzing the changes of active groups in coal oxidation at low temperatures. Spectrosc Lett. 2014;47(7):495–503.
Li JH, Li ZH, Yang YL, Wang CJ. Study on oxidation and gas release of active sites after low-temperature pyrolysis of coal. Fuel. 2018;233:237–46.
Wang J, Zhang YL, Wang JF, Zhou CS, Wu YG, Tang YB. Study on the chemical inhibition mechanism of DBHA on free radical reaction during spontaneous combustion of coal. Energy Fuels. 2020;34(5):6355–66.
Wang H, Dlugogorski BZ, Kennedy EM. Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog Energy Combust Sci. 2003;29(6):487–513.
Clemens AH, Matheson TW, Rogers DE. Low temperature oxidation studies of dried New Zealand coals. Fuel. 1991;70(2):215–21.
Wang DM, Xin HH, Qi XY, Dou GL, Qi GS, Ma LY. Reaction pathway of coal oxidation at low temperatures: a model of cyclic chain reactions and kinetic characteristics. Combust Flame. 2016;163:447–60.
Li ZH, Kong B, Wei AZ, Yang YL, Zhou YB, Zhang LJ. Free radical reaction characteristics of coal low-temperature oxidation and its inhibition method. Environ Sci Pollut Res. 2016;23:23593–605.
Shao Z, Wang D, Cao K, Si W, Liu J. Treatment of smoldering coal refuse piles: an application in China. Environ Technol. 2019;41(23):1–35.
Zhai XW, Wang TY, Li HT, Wang K, Zubíček V. Determination and predication on three zones of coal spontaneous combustion at fully-mechanised working face with nitrogen injection. Int J Oil Gas Coal T. 2019;22(3):389–416.
Yu SJ, Xie FC, Jia BY, Zhang PF. Influence study of organic and inorganic additive to coal combustion characteristic. Procedia Environ Sci. 2012;12:459–67.
Xi ZL, Li D, Feng ZY. Characteristics of polymorphic foam for inhibiting spontaneous coal combustion. Fuel. 2017;206:334–41.
Guo SL, Yan Z, Yuan SJ, Geng WL. Inhibitory effect and mechanism of l-ascorbic acid combined with tea polyphenols on coal spontaneous combustion. Energy. 2021;229:120651.
Huo YJ, Zhu HQ. Experimental and quantum chemical study on the inhibition characteristics of triphenyl phosphite to lignite oxidation at low temperature. Thermochim. Acta. 2022;717.
Lu W, Guo BL, Qi GS, Cheng WM, Yang WY. Experimental study on the effect of preinhibition temperature on the spontaneous combustion of coal based on an MgCl2 solution. Fuel. 2020;265:117032.
Slovák V, Taraba B. Urea and CaCl2 as inhibitors of coal low-temperature oxidation. J Therm Anal Calorim. 2012;110(1):363–7.
Qin BT, Dou GL, Zhong XX. Effect of stannous chloride on low-temperature oxidation reaction of coal. Fuel Process Technol. 2018;176:59–63.
Tsai YT, Yang Y, Wang CP, Shu CM, Deng J. Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Int J Energy Res. 2018;42(3):1158–71.
Cui CB, Jiang SG, Shao H, Zhang WQ, Wang K, Wu ZY. Experimental study on thermo-responsive inhibitors inhibiting coal spontaneous combustion. Fuel Process Technol. 2018;175:113–22.
Taraba B, Peter R, Slovák V. Calorimetric investigation of chemical additives affecting oxidation of coal at low temperatures. Fuel Process Technol. 2011;92(3):712–5.
Li JH, Li ZH, Yang YL, Zhang XY, Yan DC, Liu LW. Inhibitive effects of antioxidants on coal spontaneous combustion. Energy Fuels. 2017;31(12):14180–90.
Ma LY, Wang DM, Wang Y, Xin HH, Dou GL, Xu CH. Experimental investigation on a sustained release type of inhibitor for retarding the spontaneous combustion of coal. Energy Fuels. 2016;30(11):8904–14.
Wang HY, Tan B, Shao ZZ, Guo Y, Zhang ZL, Xu CF. Influence of different content of FeS2 on spontaneous combustion characteristics of coal. Fuel. 2021;288:119582.
Adamus A, Šancer J, Guřanová P, Zubiček V. An investigation of the factors associated with interpretation of mine atmosphere for spontaneous combustion in coal mines. Fuel Process Technol. 2011;92(3):663–70.
Zhang YT, Liu YR, Shi XQ, Yang CP, Wang WF, Li YQ. Risk evaluation of coal spontaneous combustion on the basis of auto-ignition temperature. Fuel. 2018;233:68–76.
Li B, Chen G, Zhang H, Sheng CD. Development of non-isothermal TGA–DSC for kinetics analysis of low temperature coal oxidation prior to ignition. Fuel. 2014;118:385–91.
Tan B, Wei HY, Zhang FC, Xu B, Chen KL. Effect of inhibitors on the thermodynamics and kinetics of spontaneous combustion of coal. J Therm Anal Calorim. 2019;140(1):295–307.
Zhu H, Zhao HR, Wei HY, Wang W, Wang HR, et al. Investigation into the thermal behavior and FTIR micro-characteristics of re-oxidation coal. Combust Flame. 2020;216:354–68.
Huang ZA, Song DH, Hu XM, Zhang YH, Gao YK, Quan SN, et al. A novel nano-modified inhibitor of tert-butyl hydroquinone/sodium polyacrylate for inhibiting coal spontaneous combustion. Energy. 2022;256:124439.
Li DT, Li W, Chen HK, Li BQ. The adjustment of hydrogen bonds and its effect on pyrolysis property of coal. Fuel Process Technol. 2004;85(8–10):815–25.
Song HJ, Liu GR, Zhang JZ, Wu JH. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process Technol. 2017;156:454–60.
Fu R, Lu T, Chen FW. Comparing methods for predicting the reactive site of electrophilic substitution. Acta Phys Chim Sin. 2014;30(4):628–39.
Lu T, Chen F. Quantitative analysis of molecular surface based on improved marching Tetrahedra algorithm. J Mol Graph Model. 2012;38:314–23.
Lu T, Chen F. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33(5):580–92.
Bruno G, Macetti G, Lo Presti L, Gatti C. Spin density topology. Molecules. 2020;25(15).
Aksnes G, Aksnes D, Ledaal T, Seip HM. Kinetics and mechanism of the reaction of Tripropyl Phosphite with water in acetonitrile. Acta Chem Scand. 1964;18(7):1623–8.
Ortuoste N, Allen NS, Papanastasiou M, McMahon A, Edge M, Johnson B, et al. Hydrolytic stability and hydrolysis reaction mechanism of bis(2,4-di-tert-butyl)pentaerythritol diphosphite (Alkanox P-24). Polym Degrad Stab. 2006;91(1):195–211.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, H., Xie, L., Huo, Y. et al. Investigation of the physicochemical inhibitors on coal spontaneous combustion based experimental and quantum chemical methods. J Therm Anal Calorim 149, 935–951 (2024). https://doi.org/10.1007/s10973-023-12778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12778-x