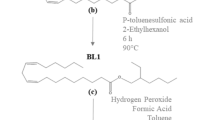
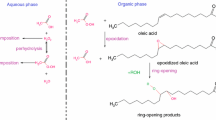
Abstract
Epoxidized soybean oil (ESO) is a sustainable alternative that reduces emissions of volatile organic compounds, enhances coating and adhesion properties, and has a wide range of applications in bio-based composites. However, the industrial-scale production of ESO through the Prilezhaev reaction presents challenges in terms of temperature regulation and the potential for uncontrolled thermal reactions. This study aimed to investigate the thermal hazards associated with ESO production using hydrogen peroxide. We analyzed the reaction mechanism and exothermic behavior using dynamic differential scanning calorimetry (DSC), accelerated calorimetry (ARC), reaction calorimetry (RClmx), and in situ Fourier transform infrared spectroscopy (FTIR). Our findings demonstrated that the addition of sulfuric acid and formic acid raised the onset decomposition temperature of hydrogen peroxide. We also identified two exothermic phases (epoxidation of the double bond and degradation of the organic matter) and observed a rapid increase in temperature and pressure, posing a safety risk. Formic acid formation and epoxidation of the double bond were the main steps of the reaction mechanism, and the exothermic behavior stems mainly from the latter. We assessed the thermal hazard level using a risk matrix and the Stoessel criterion, which highlighted the high risk of thermal runaway without proper cooling and temperature control. This paper offers practical guidance for mitigating these risks in the industry.











Similar content being viewed by others
References
Yildiz Z. Photocurable soybean oil based phosphorus containing coatings for cotton fabrics: the influence of reactive diluents. Prog Org Coat. 2023;174:107255.
Li M, Wei D, Zhang W, Liang L, Yong Q. Development of biobased polyol from epoxidized soybean oil for polyurethane anti-smudge coatings. J Appl Polym Sci. 2022;139:e53101.
Li W, Zhan Q, Yang P. Facile approach for the synthesis of performance-advantaged degradable bio-based thermoset via ring-opening metathesis polymerization from epoxidized soybean oil. ACS Sustain Chem Eng. 2023;11:1200–6.
Zheng JL, Wärnå J, Salmi T, Burel F, Taouk B, Leveneur S. Kinetic modeling strategy for an exothermic multiphase reactor system: application to vegetable oils epoxidation using P rileschajew method. AIChE J. 2016;62:726–41.
Meng Y, Taddeo F, Aguilera AF, Cai X, Russo V, Tolvanen P, Leveneur S. The Lord of the chemical rings: catalytic synthesis of important industrial epoxide compounds. Catalysts. 2021;11:765.
Knothe G, Derksen JT. Recent developments in the synthesis of fatty acid derivatives. Champaign: AOCS Press; 1999.
Petrović ZS, Zlatanić A, Lava CC, Sinadinović-Fišer S. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids—kinetics and side reactions. Eur J Lipid Sci Technol. 2002;104:293–9.
Vianello C, Piccolo D, Lorenzetti A, Salzano E, Maschio G. Study of soybean oil epoxidation: effects of sulfuric acid and the mixing program. Ind Eng Chem Res. 2018;57:11517–25.
Santacesaria E, Renken A, Russo V, Turco R, Tesser R, Di Serio M. Biphasic model describing soybean oil epoxidation with H2O2 in continuous reactors. Ind Eng Chem Res. 2012;51:8760–7.
Wu S-H, Chi J-H, Huang C-C, Lin N-K, Peng J-J, Shu C-M. Thermal hazard analyses and incompatible reaction evaluation of hydrogen peroxide by DSC. J Therm Anal Calorim. 2010;102:563–8.
Wu D, Qian X, Liu L, Zang N. Effect of inorganic salt and organic acid on the thermal runaway of hydrogen peroxide. J Loss Prev Process Ind. 2019;57:34–40.
Casson V, Maschio G. Screening analysis for hazard assessment of peroxides decomposition. Ind Eng Chem Res. 2012;51:7526–35.
Leveneur S. Thermal safety assessment through the concept of structure–reactivity: application to vegetable oil valorization. Org Process Res Dev. 2017;21:543–50.
Vianello C, Salzano E, Maschio G. Thermal behaviour of peracetic acid for the epoxydation of vegetable oils in the presence of catalyst. Process Saf Environ Prot. 2018;116:718–26.
Rakotondramaro H, Wärnå J, Estel L, Salmi T, Leveneur S. Cooling and stirring failure for semi-batch reactor: application to exothermic reactions in multiphase reactor. J Loss Prev Process Ind. 2016;43:147–57.
Zora N, Rigaux T, Buvat J-C, Lefebvre D, Leveneur S. Influence assessment of inlet parameters on thermal risk and productivity: application to the epoxidation of vegetable oils. J Loss Prev Process Ind. 2021;72:104551.
Jiang W, Ni L, Jiang J, Chen Q, Chen Z, Ye S. Thermal hazard and reaction mechanism of the preparation of adipic acid through the oxidation with hydrogen peroxide. AIChE J. 2021;67:e17089.
Bregante DT, Priyadarshini P, Flaherty DW. Kinetic and spectroscopic evidence for reaction pathways and intermediates for olefin epoxidation on Nb in* BEA. J Catal. 2017;348:75–89.
Wu H-B, Liu S-H, Cao C-R. Influence and assessment of AIBN on thermal hazard under process situations. J Therm Anal Calorim. 2021;144:1547–55.
Wang W, Fang J, Pan X, Hua M, Jiang J, Ni L, Jiang J. Thermal research on the uncontrolled behavior of styrene bulk polymerization. J Loss Prev Process Ind. 2019;57:239–44.
Zhao J, Gui X, Zhang W, Lin S, Tu Y, Hu J, He D, Huang X. Thermal hazard evaluation of styrene-methyl methacrylate bulk copolymerization by differential scanning calorimetry and accelerating rate calorimetry. Thermochim Acta. 2021;706:179052.
Zhao J, Zhang W, Hu J, Lin S, Gui X, Li S, Wang X, Li Z, Tu Y, Nian F. Research on the risk of thermal runaway in the industrial process of styrene solution polymerization. Org Process Res Dev. 2021;25:1366–74.
Stoessel F. Thermal safety of chemical processes: risk assessment and process design. New York: Wiley; 2021.
Zhang J, Shen B, Liu Z, Wang S, Zhang X. Applying experiment and numerical simulation to evaluate the thermal hazards of Bis (1-(tert-butylperoxy)-1-methylethyl)-benzene in the presence of metal ions or sulfuric acid. Thermochim Acta. 2022;717:179329.
Liu Y, Ni L, Yao H, Su J, Cheng Z. The effects of acid and hydrogen peroxide stabilizer on the thermal hazard of adipic acid green synthesis. Sustainability. 2023;15:6530.
Wu L, Chen K, Cheng S, Lee B, Shu C. Thermal decomposition of hydrogen peroxide in the presence of sulfuric acid. J Therm Anal Calorim. 2008;93:115–20.
Anothairungrat S, Ouajai S, Piyamongkala K. Screening test of evaluation thermal hazard for H2O2 by DSC. In: IOP conference series: earth and environmental science. IOP Publishing; 2019. p. 012017.
Group H. HarsBook: a technical guide for the assessment of highly reactive chemical systems. In: DECHEMA; 2002.
Muhammad Y, Shoukat A, Rahman AU, Rashid HU, Ahmad W. Oxidative desulfurization of dibenzothiophene over Fe promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 catalysts using hydrogen peroxide and formic acid as oxidants. Chin J Chem Eng. 2018;26:593–600.
Sun Y, Ni L, Papadaki M, Zhu W, Jiang J, Mashuga C, Wilhite B, Mannan MS. Process hazard evaluation for catalytic oxidation of 2-octanol with hydrogen peroxide using calorimetry techniques. Chem Eng J. 2019;378:122018.
Jalil MJ, Azmi IS, Hadi A. Highly production of dihydroxystrearic acid from catalytic epoxidation process by in situ peracid mechanism. Environ Prog Sustain Energy. 2022;41:e13764.
Moreno VC, Russo V, Tesser R, Di Serio M, Salzano E. Thermal risk in semi-batch reactors: the epoxidation of soybean oil. Process Saf Environ Prot. 2017;109:529–37.
Wai PT, Jiang P, Shen Y, Zhang P, Gu Q, Leng Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019;9:38119–36.
Zhou C, Zhang L, Yang Z, Pan Q, He Z, Wang C, Liu Y, Song S, Yang Z, Chen Y. Synthesis and characterization of carboxymethyl chitosan/epoxidized soybean oil based conjugate catalyed by UV light, and its application as drug carrier for fusarium wilt. Int J Biol Macromol. 2022;212:11–9.
Hautfenne A. Standard methods for the analysis of oils, fats and derivatives, 1st supplement: part 5 (1982) section III, glycerines. Section IV, alkaline soaps. Pure Appl Chem. 1982;54:1257–95.
Zhao X, Cheng K, Hao J, Liu D. Preparation of peracetic acid from hydrogen peroxide, part II: kinetics for spontaneous decomposition of peracetic acid in the liquid phase. J Mol Catal A Chem. 2008;284:58–68.
Shi H, Zhang Z, Wang Y. Mechanism on epoxidation of alkenes by peracids: a protonation-promoted pathway and its quantum chemical elucidation. J Mol Catal A Chem. 2005;238:13–25.
Olivieri GV, de Quadros Jr JV, Giudici R. Epoxidation reaction of soybean oil: experimental study and comprehensive kinetic modeling. Ind Eng Chem Res. 2020;59:18808–23.
Santacesaria E, Turco R, Russo V, Tesser R, Di Serio M. Soybean oil epoxidation: kinetics of the epoxide ring opening reactions. Processes. 2020;8:1134.
Campanella A, Baltanás MA. Degradation of the oxirane ring of epoxidized vegetable oils in liquid–liquid heterogeneous reaction systems. Chem Eng J. 2006;118:141–52.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (21404122) and the Guangdong Natural Science Foundation (2021A1515012334) for providing financial support.
Author information
Authors and Affiliations
Contributions
FZ contributed to conceptualization, methodology, software, investigation, and writing—original draft. YD contributed to validation, formal analysis, visualization, and software. SL contributed to writing—review and editing, and funding acquisition. XG contributed to writing—review and editing. JH contributed to writing—review and editing and funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, F., Dong, Y., Lin, S. et al. Thermal hazard and reaction mechanism for the preparation of epoxidized soybean oil from hydrogen peroxide. J Therm Anal Calorim 149, 637–651 (2024). https://doi.org/10.1007/s10973-023-12665-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12665-5