Abstract
Water hyacinth is recognized as a harmful plant, it extracts heavy metals from water bodies, and its disposal is quite challenging. The metal-impregnated samples can be effectively utilized to generate bio-oil and carbon hybrids by pyrolysis along with metal recovery in char. XRD, FESEM, and TEM analyzed the Fe/Cu-impregnated samples with an average particle size of 15.3 and 116 nm for Fe and Cu. Pyrolysis experiments were conducted at the optimum conditions to attain a maximum conversion of 68 and 48% for Fe and Cu-impregnated biomass and validated by kinetic modeling. The kinetic study of raw and metal-loaded samples was performed by modeling with Friedman, FWO, KAS, Kissinger, and Starink isoconversional processes at distinct heating rates of 10 to 25 °C min−1 to estimate optimum conditions for pyrolytic conversion. The average activation energy for raw, Fe, and Cu-impregnated samples was maximum with the Friedman method 173.9, 89.1, and 97.4 kJ mol−1, respectively. The metal-impregnated water hyacinth resulted in lower activation energy for reaction in comparison with the raw water hyacinth. The ΔHα (168.9 kJ mol−1) and ΔGα (164.9 kJ mol−1) are maximum for the raw water hyacinth in comparison with metal-impregnated samples, signifying the higher energy requirement for breaking reactant bonds. Model prediction was done by the Z-Master plot, which concluded that as the conversion (α) ≤ 0.5, the thermal degradation follows the second- and third-order reaction mechanism, and a further increase in conversion (α) > 0.5 follows the first-order reaction mechanism.
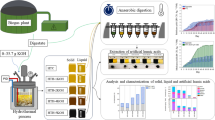
Graphical abstract










Similar content being viewed by others
Availability of data and materials
The data and materials can be made available on request.
Abbreviations
- WH:
-
Water hyacinth
- Cu-WH:
-
Cu-impregnated water hyacinth
- Fe-WH:
-
Fe-impregnated water hyacinth
- TGA:
-
Thermogravimetric analysis
- DTG:
-
Derivative thermogravimetric
- KAS:
-
Kissinger–Akahira–Sunose
- FWO:
-
Flynn–Wall–Ozawa
- DAEM:
-
Simplified distributed activation energy method
- \(E_{\alpha }\) :
-
Activation energy (kJ mol−1)
- \(A\) :
-
Frequency factor (s−1)
- \(\alpha\) :
-
Degree of conversion
- \(i\) :
-
Temperature program (°C)
- \(\beta\) :
-
Heating rate (°C min−1)
- \(\Delta H_{\alpha }\) :
-
Change in enthalpy (kJ mol−1)
- \(\Delta G_{\alpha }\) :
-
Gibbs free energy (kJ mol−1)
- \(\Delta S_{\alpha }\) :
-
Change in entropy (kJ mol−1)
References
de Caprariis B, Scarsella M, Bavasso I, Bracciale MP, Tai L, De Filippis P. Effect of Ni, Zn and Fe on hydrothermal liquefaction of cellulose: Impact on bio-crude yield and composition. J Anal Appl Pyrolysis. 2021;157: 105225. https://doi.org/10.1016/j.jaap.2021.105225.
Ibrahim M, Osman O, Mahmoud AA, Elhaes H. Spectroscopic analyses of water hyacinth: FTIR and modeling approaches. Der Pharma Chem. 2015;7:182–8.
Tajvidi K, Pupovac K, Kükrek M, Palkovits R. Copper-based catalysts for efficient valorization of cellulose. Chemsuschem. 2012;5:2139–42. https://doi.org/10.1002/cssc.201200482.
Yang J, (Sophia) He Q, Yang L. A review on hydrothermal co-liquefaction of biomass. Appl Energy. 2019;81:926–45. https://doi.org/10.1016/j.rser.2017.05.178.
Okolie JA, Nanda S, Dalai AK, Kozinski JA. Chemistry and specialty industrial applications of lignocellulosic biomass. Waste Biomass Valorization. 2021;12:2145–69. https://doi.org/10.1007/s12649-020-01123-0.
Mohan D, Pittman CU, Steele PH. Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels. 2006;20:848–89. https://doi.org/10.1021/ef0502397.
Özsin G, Pütün AE. Insights into pyrolysis and co-pyrolysis of biomass and polystyrene: thermochemical behaviors, kinetics and evolved gas analysis. Energy Convers Manag. 2017;149:675–85. https://doi.org/10.1016/j.enconman.2017.07.059.
Duan P, Xu Y, Wang F, Wang B, Yan W. Catalytic upgrading of pretreated algal bio-oil over zeolite catalysts in supercritical water. Biochem Eng J. 2016;116:105–12. https://doi.org/10.1016/j.bej.2015.12.006.
Grilc M, Veryasov G, Likozar B, Jesih A, Levec J. Hydrodeoxygenation of solvolysed lignocellulosic biomass by unsupported MoS2, MoO2, Mo2C and WS2 catalysts. Appl Catal B Environ. 2015;163:467–77. https://doi.org/10.1016/j.apcatb.2014.08.032.
Milina M, Mitchell S, Pérez-Ramírez J. Prospectives for bio-oil upgrading via esterification over zeolite catalysts. Catal Today. 2014;235:176–83. https://doi.org/10.1016/j.cattod.2014.02.047.
Wang S, Cai Q, Zhang F, Li X, Zhang L, Luo Z. Hydrogen production via catalytic reforming of the bio-oil model compounds: acetic acid, phenol and hydroxyacetone. Int J Hydrogen Energy. 2014;39:18675–87. https://doi.org/10.1016/j.ijhydene.2014.01.142.
Guna V, Ilangovan M, Anantha Prasad MG, Reddy N. Water hyacinth: a unique source for sustainable materials and products. ACS Sustain Chem Eng. 2017;5:4478–90. https://doi.org/10.1021/acssuschemeng.7b00051.
Sindhu R, Binod P, Pandey A, Madhavan A, Alphonsa JA, Vivek N. Water hyacinth a potential source for value addition: an overview. Bioresour Technol. 2017;230:152–62. https://doi.org/10.1016/j.biortech.2017.01.035.
Li F, He X, Srishti A, Song S, Tiang Wah Tan H, Sweeney DJ. Water hyacinth for energy and environmental applications: a review. Bioresour Technol. 2021;327:124809. https://doi.org/10.1016/j.biortech.2021.
Tavasoli A, Barati M, Karimi A. Conversion of sugarcane bagasse to gaseous and liquid fuels in near-critical water media using K2O promoted Cu/γ-Al2O3-MgO nanocatalysts. Biomass Bioenergy. 2015;80:63–72. https://doi.org/10.1016/j.biombioe.2015.04.031.
Kinata SE, Loubar K, Paraschiv M, Tazerout M, Belloncle C. Catalytic hydroliquefaction of charcoal CCB (copper, chromium and boron)-treated wood for bio-oil production: Influence of CCB salts, residence time and catalysts. Appl Energy. 2014;115:57–64. https://doi.org/10.1016/j.apenergy.2013.10.057.
Liu WJ, Tian K, Jiang H, Zhang XS, Ding HS, Yu HQ. Selectively improving the bio-oil quality by catalytic fast pyrolysis of heavy-metal-polluted biomass: take copper (Cu) as an example. Environ Sci Technol. 2012;46:7849–56. https://doi.org/10.1021/es204681y.
Singh R, Balagurumurthy B, Prakash A, Bhaskar T. Catalytic hydrothermal liquefaction of water hyacinth. Bioresour Technol. 2015;178:157–65. https://doi.org/10.1016/j.biortech.2014.08.119.
Zhou C, Zhu X, Qian F, Shen W, Xu H, Zhang S. Catalytic hydrothermal liquefaction of rice straw in water/ethanol mixtures for high yields of monomeric phenols using reductive CuZnAl catalyst. Fuel Process Technol. 2016;154:1–6. https://doi.org/10.1016/j.fuproc.2016.08.010.
Wang W, Yu Q, Meng H, Han W, Li J, Zhang J. Catalytic liquefaction of municipal sewage sludge over transition metal catalysts in ethanol-water co-solvent. Bioresour Technol. 2018;249:361–7. https://doi.org/10.1016/j.biortech.2017.09.205.
Lee JH, Hwang H, Moon J, Choi JW. Characterization of hydrothermal liquefaction products from coconut shell in the presence of selected transition metal chlorides. J Anal Appl Pyrolysis. 2016;122:415–21. https://doi.org/10.1016/j.jaap.2016.11.005.
Lee JH, Hwang H, Choi JW. Effects of transition metals on hydrothermal liquefaction of empty fruit bunches (EFB) for conversion to biofuel and valuable chemicals. Energy. 2018;162:1–9. https://doi.org/10.1016/j.energy.2018.07.197.
Sarkar M, Rahman AKML, Bhoumik NC. Remediation of chromium and copper on water hyacinth (E. crassipes) shoot powder. Water Resour Ind. 2017;17:1–6. https://doi.org/10.1016/j.wri.2016.12.003.
Yang S, Zhang X, Chen L, Sun L, Xie X, Zhao B. Production of syngas from pyrolysis of biomass using Fe/CaO catalysts: effect of operating conditions on the process. J Anal Appl Pyrolysis. 2017;125:1–8. https://doi.org/10.1016/j.jaap.2017.05.007.
Dhyani V, Kumar J, Bhaskar T. Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Bioresour Technol. 2017;245:1122–9. https://doi.org/10.1016/j.biortech.2017.08.189.
Zhu H, Liu N. Kinetic analysis based on the kinetic compensation effect and optimization calculation. Thermochim Acta. 2020;690: 178686. https://doi.org/10.1016/j.tca.2020.178686.
Cai J, Xu D, Dong Z, Yu X, Yang Y, Banks SW. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk. Renew Sust Energ Rev. 2018;82:2705–15. https://doi.org/10.1016/j.rser.2017.09.113.
Martín-lara MÁ, Iáñez-rodríguez I, Blázquez G, Quesada L, Pérez A. Kinetics of thermal decomposition of some biomasses in an inert environment. An investigation of the effect of lead loaded by biosorption. Waste Manage. 2017;70:101–13. https://doi.org/10.1016/j.wasman.2017.09.021.
Soria-verdugo A, Goos E, García-hernando N, Riedel U. Analyzing the pyrolysis kinetics of several microalgae species by various di ff erential and integral isoconversional kinetic methods and the distributed activation energy model. Algal Res. 2018;32:11–29. https://doi.org/10.1016/j.algal.2018.03.005.
Tabal A, Barakat A, Aboulkas A, El K. Pyrolysis of ficus nitida wood: determination of kinetic and thermodynamic parameters. Fuel. 2021;283: 119253. https://doi.org/10.1016/j.fuel.2020.119253.
Hihu Muigai H, Choudhury BJ, Kalita P, Moholkar VS. Physico–chemical characterization and pyrolysis kinetics of Eichhornia Crassipes, Thevetia Peruviana, and Saccharum Officinarum. Fuel. 2021;289: 119949. https://doi.org/10.1016/j.fuel.2020.119949.
Cortés AM, Bridgwater AV. Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process Technol. 2015;138:184–93. https://doi.org/10.1016/j.fuproc.2015.05.013.
Ma P, Yang J, Xing X, Weihrich S, Fan F, Zhang X. Isoconversional kinetics and characteristics of combustion on hydrothermally treated biomass. Renew Energ. 2017;114:1069–76. https://doi.org/10.1016/j.renene.2017.07.115.
Huang L, Liu J, He Y, Sun S, Chen J, Sun J. Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel. Bioresour Technol. 2016;218:631–42. https://doi.org/10.1016/j.biortech.2016.06.133.
Chandrasekaran A, Ramachandran S, Subbiah S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis. Bioresour Technol. 2017;233:413–22. https://doi.org/10.1016/j.biortech.2017.02.119.
Mallick D, Bora BJ, Baruah D, Barbhuiya SA, Banik R, Garg J. Mechanistic investigation and thermal degradation of eichhornia crassipes using thermogravimetric analysis. SSRN Electron J. 2020. https://doi.org/10.2139/ssrn.3668845.
Kumar SAP, Nagarajan R, Prasad KM, Anand B. Thermogravimetric study and kinetics of banana peel pyrolysis : a comparison of ‘model-free’ methods. Biofuels. 2019;0:1–10. https://doi.org/10.1080/17597269.2019.1647375.
Yadav P, Reddy SN. Hydrothermal liquefaction of Fe-impregnated water hyacinth for generation of liquid bio-fuels and nano Fe carbon hybrids. Bioresour Technol. 2020;313: 123691. https://doi.org/10.1016/j.biortech.2020.123691.
Rotaru R, Savin M, Tudorachi N, Peptu C, Samoila P, Sacarescu L. Ferromagnetic iron oxide-cellulose nanocomposites prepared by ultrasonication. Polym Chem Roy Soc Chem. 2018;9:860–8. https://doi.org/10.1039/C7PY01587A.
Wauton I, Ogbeide SE. Determination of the activation energy of water hyacinth (Eichornia crassipes) pyrolysis. Int J Green Energy. 2019;16:1571–6. https://doi.org/10.1080/15435075.2019.1677236.
Zhang B, Lin Q, Zhang Q, Wu K, Pu W, Yang M. Catalytic hydrothermal liquefaction of Euglena sp. microalgae over zeolite catalysts for the production of bio-oil. RSC Adv Roy Soc Chem. 2017;7:8944–51. https://doi.org/10.1039/C6RA28747F.
Gollakota ARK, Kishore N, Gu S. A review on hydrothermal liquefaction of biomass. Renew Sust Energ Rev. 2018;81:1378–92. https://doi.org/10.1016/j.rser.2017.05.178.
Yang X, Zhao Y, Li R, Wu Y, Yang M. A modified kinetic analysis method of cellulose pyrolysis based on TG–FTIR technique. Thermochim Acta. 2018;665:20–7. https://doi.org/10.1016/j.tca.2018.05.008.
Zhong S, Zhang B, Liu C, Shujaa aldeen A. Mechanism of synergistic effects and kinetics analysis in catalytic co-pyrolysis of water hyacinth and HDPE. Energy Convers Manag. Elsevier Ltd. 2021;228:113717. https://doi.org/10.1016/j.enconman.2020.113717.
Vuppaladadiyam AK, Zhao M, Memon MZ, Soomro AF. Microalgae as a renewable fuel resource: A comparative study on the thermogravimetric and kinetic behavior of four microalgae. Sustain Energy Fuels Royal Soc Chem. 2019;3:1283–96. https://doi.org/10.1039/C9SE00114J.
Zadeh ZE, Abdulkhani A, Aboelazayem O, Saha B. Recent insights into lignocellulosic biomass pyrolysis: a critical review on pretreatment, characterization, and products upgrading. Processes. 2020;8:31. https://doi.org/10.3390/pr8070799.
Shao J, Yan R, Chen H, Yang H, Ho D. Catalytic effect of metal oxides on pyrolysis of sewage sludge. Fuel Process Technol. 2010;91:1113–8. https://doi.org/10.1016/j.fuproc.2010.03.023.
Liu Y, Guo F, Li X, Li T, Peng K, Guo C. Catalytic effect of iron and nickel on gas formation from fast biomass pyrolysis in a micro fluidized bed reactor: a kinetic study. Energy Fuels. 2017;12278–87. https://doi.org/10.1021/acs.energyfuels.7b02214.
Tran TK, Kim N, Leu HJ, Pham MP, Luong NA, Vo HK. The production of hydrogen gas from modified water hyacinth (Eichhornia Crassipes) biomass through pyrolysis process. Int J Hydrogen Energy. 2021;46:13976–84. https://doi.org/10.1016/j.ijhydene.2020.08.225.
Gulab H, Hussain K, Malik S, Hussain M. Effect of process conditions on bio-oil composition and production from catalytic pyrolysis of water hyacinth biomass. Waste Biomass Valorization. 2019;10:2595–609. https://doi.org/10.1007/s12649-018-0238-5.
He J, Strezov V, Kan T, Weldekidan H, Asumadu-Sarkodie S, Kumar R. Effect of temperature on heavy metal(loid) deportment during pyrolysis of Avicennia marina biomass obtained from phytoremediation. Bioresour Technol. 2019;278:214–22. https://doi.org/10.1016/j.biortech.2019.01.101.
Funding
The researchers are thankful to Science and Engineering Research Board, Government of India (Grant Number: CRG/2020/000360) for the financial support to perform the experiments under Core Research Grant.
Author information
Authors and Affiliations
Contributions
PY: She is a Ph.D. Student who have conducted experiments, kinetics and thermogravimetric study, analysis of the obtained data, preparation of figures and tables along with the manuscript. SNR: Development of concept, pyrolysis, Analysis of the generated data, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, P., Reddy, S.N. Experimental and Kinetic modeling of In-situ Catalytic (Fe/Cu) Pyrolytic Degradation of Water Hyacinth. J Therm Anal Calorim 148, 12761–12780 (2023). https://doi.org/10.1007/s10973-023-12573-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12573-8