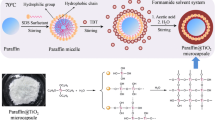
Abstract
Paraffin- and titania (TiO2)-based nano-encapsulated phase change materials (NePCMs) were synthesized by the sol–gel process to improve thermal as well as phase change performance. Nine different samples of NePCMs were prepared by varying core/shell mass ratios, and their effect was investigated on thermal performance. The paraffin/TiO2 nanocapsules were characterized by Fourier transform infrared spectrophotometer (FTIR), scanning electron microscopy (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), energy-dispersive X-ray (EDX), and dynamic light scattering (DLS). The characterization techniques confirmed that the paraffin was successfully contained in the TiO2 shell. The maximum effective melting (73.47 Jg−1) and solidification (71.87 Jg−1) latent heat for the NePCMs were found to be at a 52.33% encapsulation ratio. The mean size of the NePCMs was approximately 238 nm measured from DLS. The thermal properties of the same sample stayed almost the same even after 100 thermal cycles validated by the differential scanning calorimetry (DSC) curves, and thermal stability was evaluated by a thermogravimetric analyzer (TGA).
Graphical abstract















Similar content being viewed by others
Data and code availability
Not applicable.
Abbreviations
- e:
-
Encapsulation efficiency
- r :
-
Encapsulation ratio
- \(\Delta H_{{\text{m,NePCM}}}\) :
-
Melting latent heat of the nano-encapsulated phase change material
- \(\Delta H_{{\text{m,PCM}}}\) :
-
Melting latent heat of the bulk phase change material
- NePCMs:
-
Nano-encapsulated phase change materials
- PCMs:
-
Phase change materials
- SiO2 :
-
Silica
- SDS:
-
Sodium dodecyl sulfate
- \(\Delta H_{{\text{s,NePCM}}}\) :
-
Solidification latent heat of the nano-encapsulated phase change material
- \(\Delta H_{{\text{s,PCM}}}\) :
-
Solidification latent heat of the bulk phase change material
- TiO2 :
-
Titania
- TBT:
-
Tetrabutyl orthotitanate
References
Fleischer A.S. Thermal energy storage using phase change materials: fundamentals and applications. 2015: Springer.
Jamekhorshid A, Sadrameli S, Farid M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew Sustain Energy Rev. 2014;31:531–42.
Faghiri S, et al. The impingement of liquid boiling droplet onto a molten phase change material as a direct-contact solidification method. Thermal Sci Eng Progress. 2021;23: 100888.
Faghiri S, et al. Multi-objective optimization of multiple droplet impacts on a molten PCM using NSGA-II optimizer and artificial neural network. Sci Rep. 2023;13(1):10543.
Poureslami P., Faghiri S, Shafii, M. B. Simultaneous double droplet impact on a molten phase change material pool: An experimental investigation. Phys Fluids. 2023;35(2).
Alkan C, Sarı A, Karaipekli A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Convers Manage. 2011;52(1):687–92.
Keyan K., et al., Microencapsulation of PCMs in textiles: a review. J Textile Apparel Technol Manage. 2012;7(3).
Su J-F, et al. Fabrication and properties of microencapsulated-paraffin/gypsum-matrix building materials for thermal energy storage. Energy Convers Manage. 2012;55:101–7.
Li W, et al. Nano-encapsulated phase change material slurry (Nano-PCMS) saturated in metal foam: a new stable and efficient strategy for passive thermal management. Energy. 2018;165:743–51.
Ho C, et al. Forced convection heat transfer of Nano-Encapsulated Phase Change Material (NEPCM) suspension in a mini-channel heatsink. Int J Heat Mass Transf. 2020;155: 119858.
Li J, et al. Micro-encapsulated paraffin/high-density polyethylene/wood flour composite as form-stable phase change material for thermal energy storage. Sol Energy Mater Sol Cells. 2009;93(10):1761–7.
Pan L, et al. Preparation, characterization and thermal properties of micro-encapsulated phase change materials. Sol Energy Mater Sol Cells. 2012;98:66–70.
Qiu X, et al. Microencapsulated n-octadecane with different methylmethacrylate-based copolymer shells as phase change materials for thermal energy storage. Energy. 2012;46(1):188–99.
Li W, et al. Composition and characterization of thermoregulated fiber containing acrylic-based copolymer microencapsulated phase-change materials (MicroPCMs). Ind Eng Chem Res. 2014;53(13):5413–20.
Li B, et al. Fabrication and properties of microencapsulated paraffin@ SiO2 phase change composite for thermal energy storage. ACS Sustain Chem Eng. 2013;1(3):374–80.
Salunkhe PB, Shembekar PS. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew Sustain Energy Rev. 2012;16(8):5603–16.
Sánchez-Silva L, et al. Poly (urea-formaldehyde) microcapsules containing commercial paraffin: in situ polymerization study. Colloid Polym Sci. 2018;296(9):1449–57.
Shi J, et al. Nano-encapsulated phase change materials prepared by one-step interfacial polymerization for thermal energy storage. Mater Chem Phys. 2019;231:244–51.
Hawlader M, Uddin M, Khin MM. Microencapsulated PCM thermal-energy storage system. Appl Energy. 2003;74(1–2):195–202.
Zhang H, Wang X, Wu D. Silica encapsulation of n-octadecane via sol–gel process: a novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J Colloid Interface Sci. 2010;343(1):246–55.
Ji W, et al. Self-assembly fabrication of GO/TiO2@ paraffin microcapsules for enhancement of thermal energy storage. Powder Technol. 2021;385:546–56.
Ishak S, et al. Effect of core-shell ratio on the thermal energy storage capacity of SiO2 encapsulated lauric acid. J Energy Storage. 2021;42: 103029.
Wang H, et al. Novel synthesis of silica coated palmitic acid nanocapsules for thermal energy storage. J Energy Storage. 2020;30: 101402.
Ishak S, et al. Microencapsulation of stearic acid with SiO2 shell as phase change material for potential energy storage. Sci Rep. 2020;10(1):1–15.
Ghufran M, Huitink D. Synthesis of nano-size paraffin/silica-based encapsulated phase change materials of high encapsulation ratio via sol–gel method. J Mater Sci. 2023;1–17.
Genc M, Karagoz Genc Z. Microencapsulated myristic acid–fly ash with TiO2 shell as a novel phase change material for building application. J Thermal Anal Calorimetry. 2018;131(3):2373–80.
Zhao L, et al. Fabrication and properties of microencapsulated n-octadecane with TiO2 shell as thermal energy storage materials. Sol Energy. 2016;127:28–35.
Fan X, et al. Full-spectrum light-driven phase change microcapsules modified by CuS-GO nanoconverter for enhancing solar energy conversion and storage capability. Sol Energy Mater Sol Cells. 2021;223: 110937.
Fan X, et al. Fabrication and characteristics of solar-driven phase change microcapsules with crystalline TiO2/CuS hybrid shell for solar energy conversion and storage. J Mater Res. 2020;35(16):2126–37.
Li C, et al. Synthesis of microencapsulated stearic acid with amorphous TiO2 as shape-stabilized PCMs for thermal energy storage. Energy Procedia. 2018;152:390–4.
Latibari ST, et al. Facile synthesis and thermal performances of stearic acid/titania core/shell nanocapsules by sol–gel method. Energy. 2015;85:635–44.
Rudyak VY, Minakov A, Pryazhnikov M. Thermal properties of nanofluids and their similarity criteria. Tech Phys Lett. 2017;43:23–6.
Li M, Liu J, Shi J. Synthesis and properties of phase change microcapsule with SiO2-TiO2 hybrid shell. Sol Energy. 2018;167:158–64.
Latibari ST, et al. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy. 2013;61:664–72.
Yuan H, et al. Effect of alkaline pH on formation of lauric acid/SiO2 nanocapsules via sol-gel process for solar energy storage. Sol Energy. 2019;185:374–86.
Sarı A, Karaipekli A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol Energy Mater Sol Cells. 2009;93(5):571–6.
Sarı A, et al. Microencapsulated n-octacosane as phase change material for thermal energy storage. Sol Energy. 2009;83(10):1757–63.
Zhang H, Wang X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol Energy Mater Sol Cells. 2009;93(8):1366–76.
Dhivya S, et al. Experimental study on microcapsules of Ag doped ZnO nanomaterials enhanced Oleic-Myristic acid eutectic PCM for thermal energy storage. Thermochim Acta. 2019;671:70–82.
Ma X, et al. Synthesis and characterization of microencapsulated paraffin with TiO2 shell as thermal energy storage materials. J Mater Sci Mater Electron. 2018;29(17):15241–8.
Acknowledgements
We would like to thank Dr. Mourad Benamara at the University of Arkansas to help in the testing of the samples.
Author information
Authors and Affiliations
Contributions
MG helped in conceptualization, investigation, experimentation, writing—original draft, and visualization. DH contributed to writing—review and editing, supervision, and resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghufran, M., Huitink, D. Synthesis and thermal performance of nano-sized paraffin-based titania encapsulated PCMs via sol–gel method. J Therm Anal Calorim 148, 11629–11640 (2023). https://doi.org/10.1007/s10973-023-12519-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12519-0