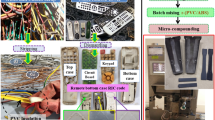
Abstract
Industrial waste disposal is one of the major environmental concerns faced by all nations today. The present work describes the thermal degradation kinetics of polyurethane rich shoe sole waste (PUW) filled natural rubber (NR) composites and their transport behavior in various solvents. NR composites with PUW were prepared by incorporating it in the range 0–10 phr (parts per hundred rubber). The thermal behavior of the NR-PUW composites was researched by thermogravimetric analysis (TGA) at 30–600 °C. Kinetics of thermal degradation was studied by plotting thermogravimetric curve at four distinct heating rates, viz., 7.5, 10, 15, 20 °C min−1. The energy of activation (Ea) calculated using Flynn–Wall–Oszaka (FWO), Kissinger–Akahira–Sunose (KAS), Tang and Starink models were found to be well correlated. The NR composite with 7.5 phr PUW showed higher Ea proving its better thermal stability compared to other composites. Multi-step degradation kinetics of the composites was evidenced by the variation in energy of activation as conversion increases. The solvent transport behavior of the composites was investigated in toluene, xylene, hexane, and petrol. Swelling was found to be maximum for toluene and solvent uptake decreases with further PUW loading. The diffusion parameters of the composites exhibited a decreasing trend as PUW increases.
Graphical abstract











Similar content being viewed by others
References
Kurian T, Mathew NM. Natural rubber: production, properties and applications. Biopolym Biomed Environ Appl. 2011;70:403–36.
Phinyocheep P. 3 - Chemical modification of natural rubber (NR) for improved performance. In: Kohjiya S, Ikeda Y, editors. Chemistry, manufacture and applications of natural rubber. Woodhead Publishing; 2014. p. 68–118.
Zou Y, Sun Y, Zhang Y, He J, Tang Z, Zhu L, et al. Antioxidative behavior of a novel samarium complex in styrene-butadiene rubber/silica composites. Polym Degrad Stab. 2016;133:201–10.
Robertson CG, Hardman NJ. Nature of carbon black reinforcement of rubber: perspective on the original polymer nanocomposite. Polymers. 2021;13:538.
El-Sabbagh SH, Ahmed NM, Mahmoud DS, Mohamed WS. Silica and modified silica fume waste (mSF) as reinforcing fillers for rubber industry. Pigm Resin Technol. 2020;50:74–84.
Abraham E, Elbi PA, Deepa B, Jyotishkumar P, Pothen LA, Narine SS, et al. X-ray diffraction and biodegradation analysis of green composites of natural rubber/nanocellulose. Polym Degrad Stab. 2012;97:2378–87.
Daud S, Lik OYH, Azura AR. The effects of nano-cellulose filler on tensile and thermal properties of natural rubber latex films. In: AIP Conference Proceedings. 2020. p. 2267.
Chittella H, Yoon LW, Ramarad S, Lai Z-W. Rubber waste management: a review on methods, mechanism, and prospects. Polym Degrad Stab. 2021;194:109761.
Roy K, Debnath SC, Bansod ND, Pongwisuthiruchte A, Wasanapiarnpong T, Potiyaraj P. Possible use of gypsum waste from ceramics industry as semi-reinforcing filler in epoxidized natural rubber composites. J Mater Cycl Waste Manag. 2020;22:285–94. https://doi.org/10.1007/s10163-019-00939-w.
Ren X, Sancaktar E. Use of fly ash as eco-friendly filler in synthetic rubber for tire applications. J Clean Prod. 2019;206:374–82. https://doi.org/10.1016/j.jclepro.2018.09.202.
Karkalic R, Radulovic J, Jovanovic D. Characteristics of polyurethane and elastomer parts for shoe industry produced by liquid injection molding technology. Vojnoteh Glas. 2017;65:948–67.
Staikos T, Rahimifard S. Post-consumer waste management issues in the footwear industry. Proc Inst Mech Eng Part B J Eng Manuf. 2007;221:363–8.
Kemona A, Piotrowska M. Polyurethane recycling and disposal: methods and prospects. Polymers. 2020;12:1752.
Bashpa P, Bijudas K, Dileep P, Elanthikkal S, Francis T. Reutilization of polyurethane-based shoe sole scrap as a reinforcing filler in natural rubber for the development of high-performance composites. J Elast Plast. 2022. https://doi.org/10.1177/00952443221108514.
Vyazovkin S. Kissinger method in kinetics of materials: things to beware and be aware of. Molecules. 2020;25:2813.
Zhang X. Applications of kinetic methods in thermal analysis: a review. Eng Sci. 2021;14:1–13.
Venkatesh M, Ravi P, Tewari SP. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs Flynn–Wall–Ozawa method. J Phys Chem A. 2013;117:10162–9. https://doi.org/10.1021/jp407526r.
Bafna S. On the use of Ozawa/Flynn/Wall method to determine aging activation energy for elastomers. J Elast Plast. 2021;54:56–66. https://doi.org/10.1177/00952443211020330.
Muraleedharan K, Alikutty P, Abdul Mujeeb VM, Sarada K. Kinetic studies on the thermal dehydration and degradation of Chitosan and Citralidene Chitosan. J Polym Environ. 2015;23:1–10.
Sujith A, Unnikrishnan G, Radhakrishnan CK, Padmini M. Interaction of silica and carbon black fillers with natural rubber/poly(ethylene-co-vinyl acetate) matrix by swelling studies. Polym Compos. 2007;28:705–12. https://doi.org/10.1002/pc.20340.
Visakh PM, Thomas S, Oksman K, Mathew AP. Cellulose nanofibres and cellulose nanowhiskers based natural rubber composites: diffusion, sorption, and permeation of aromatic organic solvents. J Appl Polym Sci. 2012;124:1614–23. https://doi.org/10.1002/app.35176.
Janković B, Mentus S, Jelić D. A kinetic study of non-isothermal decomposition process of anhydrous nickel nitrate under air atmosphere. Phys B Condens Matter. 2009;404:2263–9.
Moussout H, Ahlafi H, Aazza M, Bourakhouadar M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym Degrad Stab. 2016;130:1–9.
Motaung TE, Luyt AS, Bondioli F, Messori M, Saladino ML, Spinella A, et al. PMMA-titania nanocomposites: properties and thermal degradation behaviour. Polym Degrad Stab. 2012;97:1325–33.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand Sect A Phys Chem. 1966;70A:487–523.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6. https://doi.org/10.1021/ac60131a045.
Tang W, Liu Y, Zhang H, Wang C. New approximate formula for Arrhenius temperature integral. Thermochim Acta. 2003;408:39–43.
Budrugeac P, Segal E. On the Li and Tang’s isoconversional method for kinetic analysis of solid-state reactions from thermoanalytical data. J Mater Sci. 2001;36:2707–10.
Starink MJ, van Mourik P. Cooling and heating rate dependence of precipitation in an Al–Cu alloy. Mater Sci Eng A. 1992;156:183–94.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92. https://doi.org/10.1002/app.1961.070051506.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Trovati G, Sanches EA, Neto SC, Mascarenhas YP, Chierice GO. Characterization of polyurethane resins by FTIR, TGA, and XRD. J Appl Polym Sci. 2010;115:263–8. https://doi.org/10.1002/app.31096.
Intapun J, Rungruang T, Suchat S, Cherdchim B, Hiziroglu S. The Characteristics of natural rubber composites with klason lignin as a green reinforcing filler: thermal stability, mechanical and dynamical properties. Polymers. 2021;13:1109.
Shufen L, Zhi J, Kaijun Y, Shuqin Y, Chow WK. Studies on the thermal behavior of polyurethanes. Polym Plast Technol Eng. 2006;45:95–108. https://doi.org/10.1080/03602550500373634.
Danielle Galiani P, Antonio Malmonge J, Guenther Soares B, Henrique Capparelli Mattoso L. Studies on thermal–oxidative degradation behaviours of raw natural rubber: PRI and thermogravimetry analysis. Plast Rubber Compos. 2013;42:334–9. https://doi.org/10.1179/1743289811Y.0000000046.
Li S-D, Kong L, Zhong J-P. Thermal degradation kinetics and morphology of natural rubber/silica nanocomposites. J Nanosci Nanotechnol. 2006;6:541–6.
Li C, Zhong J, Yang L, Li S, Kong L, Hou T. Studies on the properties and the thermal decomposition kinetics of natural rubber prepared with calcium chloride. e-Polymers. 2010;10:72. https://doi.org/10.1515/epoly.2010.10.1.789.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32. https://doi.org/10.1002/marc.200600404.
Rajeshwari PM, Dey TK. Thermogravimetric kinetics of degradation of HDPE based MWCNTs reinforced composites. Int J Plast Technol. 2014;18:294–320.
Balan AK, Sreejith MP, Shaniba V, Jinitha T V, Subair N, Purushothaman E. Transport behavior of aromatic hydrocarbons through coconut shell powder filled thermoplastic polyurethane/natural rubber blend-composites. In: AIP Conference Proceedings; 2017. p. 20046. https://doi.org/10.1063/1.4984193
Maria H, Lyczko N, Nzihou A, Mathew C, George S, Joseph K, et al. Transport of organic solvents through natural rubber/nitrile rubber/organically modified montmorillonite nanocomposites. J Mater Sci. 2013;48:5373–86.
Mathew L, Joseph KU, Joseph R. Swelling behaviour of isora/natural rubber composites in oils used in automobiles. Bull Mater Sci. 2006;29:91–9. https://doi.org/10.1007/BF02709362.
Al Minnath M, Unnikrishnan G, Purushothaman E. Transport studies of thermoplastic polyurethane/natural rubber (TPU/NR) blends. J Membr Sci. 2011;379:361–9.
Acknowledgements
We thankfully acknowledge KSCSTE and DBT-STAR for the instrumentation facility at Department of Chemistry, St. Joseph's College (Autonomous) Devagiri, Kozhikode, affiliated to University of Calicut and J. J. Murphy Research Centre, Rubber Park India (P) Ltd., Valayanchirangara for thermal analysis and mixing, respectively. This reported work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
St. Joseph’s College and N. S. S. College Affiliated to University of Calicut.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bashpa, P., Stephy, A., Bijudas, K. et al. Thermal degradation kinetics and solvent transport behavior of natural rubber composites filled with polyurethane rich shoe sole waste from footwear industry. J Therm Anal Calorim 148, 10871–10883 (2023). https://doi.org/10.1007/s10973-023-12425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12425-5