Abstract
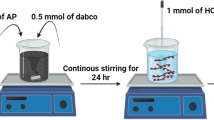
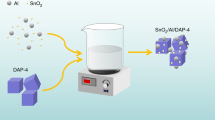
Co3O4 nanoclusters were prepared by the gas phase precipitation method. The heating pyrolysis characteristics of molecular perovskite-based energetic material (H2dabco)[NH4(ClO4)3] (DAP-4) by using Co3O4 nanocluster catalysts was studied. Results showed that when 5mass% Co3O4 added, the initial pyrolysis temperature and the DTG peak temperature of DAP-4 were reduced to 256.8 ℃ and 340 ℃, respectively, at the heating rate of 10 K min−1. When the average particle size (D50) of Co3O4 was 694 nm, the activation energy (Ea) required for heating catalytic pyrolysis of DAP-4 with the presence of Co3O4 decreased to 140.8 kJ mol−1 (Kissinger’s method) and 143.6 kJ mol−1 (Ozawa’s method). The heating catalytic pyrolysis mechanism of Co3O4 nanoclusters for the heating pyrolysis of DAP-4 was proposed. This work is helpful to understand further the heating catalytic pyrolysis characteristics of molecular perovskite-based energetic materials with metal oxide nanoclusters.










Similar content being viewed by others
References
Chen SL, Yang ZR, Wang BJ, et al. Molecular perovskite high-energetic materials. Sci China Mater. 2018;61:1123–8.
Deng P, Wang HX, Yang XB, Ren H, Jiao QJ. Thermal decomposition and combustion performance of high-energy ammonium perchlorate-based molecular perovskite. J Alloys Compd. 2020;827:154257.
Deng P, Chen PW, Fang H, Liu R, Guo XY. The combustion behavior of boron particles by using molecular perovskite energetic materials as high-energy oxidants. Combust Flame. 2022;241:112118.
Wang YM, Zhou JQ, Deng P, Guo XY, Chen PW, Liu R. Enhanced the combustion performance of DAP-4 with assembled Cu2O nanoclusters. Vacuum. 2023;208:111718.
Letsholathebe D, Thema FT, Mphale K, et al. Optical and structural stability of Co3O4 nanoparticles for photocatalytic applications. Mater Today: Proc. 2020;36:499–503.
Mahmood ZH, Jarosova M, Kzar HH, et al. Synthesis and characterization of Co3O4 nanoparticles: application as performing anode in Li-ion batteries. J Chin Chem Soc. 2022;69:657–62.
Zoromba MS, Hosny NM. Synthesis of Fe2O3, Co3O4 and NiO nanoparticles by thermal decomposition of doped polyaniline precursors. J Therm Anal Calorim. 2015;119(1):605–11.
Nusrath K, Muraleedharan K. Effect of nano-transition metal oxides of Fe, Co and Ni and ferrites of Co and Ni on the multistage thermal decomposition of oxalates of Ce(III). J Therm Anal Calorim. 2019;136(2):549–63.
Seyed G, Seyed J, Khosro B, Azam G. The effect of average particle size of nano-Co3O4 on the catalytic thermal decomposition of ammonium perchlorate particles. J Therm Anal Calorim. 2016;124:1243–54.
Tang WQ, Ren H, Jiao QJ. Synthesis of an ultra-fine Co3O4/graphene composite by one-step hydrothermal process and its effective catalytic performance on thermal decomposition of ammonium perchlorate. Ferroelectrics. 2018;527:119–32.
Yang YP, Huang KL, Liu RS, Wang LP, Zeng WW, Zhang PM. Shape-controlled synthesis of nanocubic Co3O4 by hydrothermal oxidation method. Trans Nonferrous Met Soc China. 2007;17:1082–6.
Cole KM, Kirk D, Thorpe SJ. Co3O4 nanoparticles characterized by XPS and UPS. Surf Sci Spectra. 2021;28:014001.
Mohamed AR, Li N, Sohaimi K, et al. Kinetic parameters of non-isothermal thermogravimetric non-catalytic and catalytic pyrolysis of empty fruit bunch with alumina by Kissinger and Ozawa methods. In: IOP Conference series: materials science and engineering. 2018, vol. 318, p. 012019.
Shiue GY, Huang AC, Chen JR. Thermal decomposition of triacetone triperoxide by differential scanning calorimetry. J Therm Anal Calorim. 2018;133(1):745–51.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 22175026), the BIT Research and Innovation Promoting Project (Grant No. 2022YCXY049) and Zhejiang Provincial Construction and Scientific Research Project (2021K213). Authors thank Xi’an Modern Chemistry Research Institute and North university of China for support and help.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, H., Zhang, B., Han, K. et al. Thermal decomposition and combustion characteristics of molecular perovskite energetic material DAP-4 catalyzed by Co3O4 nanoclusters. J Therm Anal Calorim 148, 10441–10448 (2023). https://doi.org/10.1007/s10973-023-12357-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12357-0