Abstract
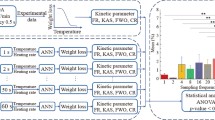
During the pyrolysis process, solid fuels decompose at different degradation rates and temperature ranges owing to their complex structure and composition. Therefore, predicting the thermal degradation of a wide range of solid fuels is paramount for understanding the thermal stability of fuels to effectively utilize them. In this study, the proposed artificial neural network (ANN) (NN-5-22-22-2), with three layers and tansig–logsig transfer functions, predicts the thermogravimetry (TG) and the derivative thermogravimetry (DTG) curves based on 24,750 experimental data points as input parameters (six different heating rates, 825 temperature points, and moisture, volatile, and fixed carbon content of five types of samples). In this exploratory study, we applied a nonlinear optimization method, namely the generalized reduced gradient (GRG) algorithm, to determine the activation energy (E) and frequency factor (A) in a nonlinear equation instead of using the conventional slope and intercept method. Five types of rank fuels were calculated using a distributed activation energy model (DAEM) at six heating rates (5, 10, 20, 30, 40, and 50 °C min−1). The results of the highest average correlation factor, low mean square error values, and low normalized mean square error values of the prediction and experimental data were in agreement. Thus, we demonstrated that the GRG algorithm is an appropriate method for analyzing kinetic data.










Similar content being viewed by others
References
Association WB. Global bioenergy statistics; 2020 [Internet]
Mohd Idris MN, Hashim H. Integrating palm oil biomass waste utilization in coal-fired power plants for meeting near-term emission targets. J Environ Manage. 2021;296:113118.
Couhert C, Commandre JM, Salvador S. Is it possible to predict gas yields of any biomass after rapid pyrolysis at high temperature from its composition in cellulose, hemicellulose and lignin? Fuel. 2009;88:408–17.
Song Y, Hu J, Liu J, Evrendilek FBM, Buyukada M. CO2-assisted co-pyrolysis of textile dyeing sludge and hyperaccumulator biomass: dynamic and comparative analyses of evolved gases, bio-oils, biochars, and reaction mechanisms. J Hazard Mater. 2020;400:123190.
Zhang J, Zou H, Liu J, Evrendilek F, Xie W, He Y, et al. Comparative (co-)pyrolytic performances and by-products of textile dyeing sludge and cattle manure: deeper insights from Py-GC/MS, TG-FTIR, 2D-COS and PCA analyses. J Hazard Mater. 2021;401:123276.
Alam M, Rammohan D, Bhavanam A, Peela NR. Wet torrefaction of bamboo saw dust and its co-pyrolysis with plastic. Fuel. 2021;285:119188.
Xu G, Cai X, Wang S, Fang B, Wang H, Zhu Y. Characteristics, kinetics, infrared analysis and process optimization of co-pyrolysis of waste tires and oily sludge. J Environ Manage. 2022;316:115278.
Xu G, Cai X, Wang L, Zhang Q, Fang B, Zhong X, et al. Thermogravimetric-infrared analysis and performance optimization of co-pyrolysis of oily sludge and rice husks. Int J Hydrog Energy. 2022;47:27437–51.
Tsekos C, Tandurella S, de Jong W. Estimation of lignocellulosic biomass pyrolysis product yields using artificial neural networks. J Anal Appl Pyrol. 2021;157:105180.
Xiao R, Yang W, Cong X, Dong K, Xu J, Wang D, et al. Thermogravimetric analysis and reaction kinetics of lignocellulosic biomass pyrolysis. Energy. 2020;201:117537.
Weisenberger MC, Burgess J, Schobert HH, Hower JC. Thermal properties of Pennsylvania anthracite. Fuel. 2020;266:117101.
Nyoni B, Duma S, Shabangy SV, Hlangothi SP. Comparison of the slow pyrolysis behavior and kinetics of coal, wood and algae at high heating rates. Nat Resour Res. 2020;29:3943–55.
Sunphorka S, Chalermsinsuwan B, Piumsomboon P. Artificial neural network model for the prediction of kinetic parameters of biomass pyrolysis from its constituents. Fuel. 2017;193:142–58.
Sathiya Prabhakaran SP, Swaminathan G, Joshi VV. Thermogravimetric analysis of hazardous waste: Pet-coke, by kinetic models and artificial neural network modeling. Fuel. 2021;287:119470.
Bong JT, Loy ACM, Chin BLF, Lam MK, Tang DKH, Lim HY, et al. Artificial neural network approach for co-pyrolysis of Chlorella vulgaris and peanut shell binary mixtures using microalgae ash catalyst. Energy. 2020;207:118289.
Ni Z, Bi H, Jiang C, Wang C, Tian J, Zhou W, et al. Investigation of the co-pyrolysis of coal slime and coffee industry residue based on machine learning methods and TG-FTIR: Synergistic effect, kinetics and thermodynamic. Fuel. 2021;305:121527.
Liew JX, Loy ACM, Chin BLF, AlNouss A, Shahbaz M, Al-Ansari T, et al. Synergistic effects of catalytic co-pyrolysis of corn cob and HDPE waste mixtures using weight average global process model. Renew Energy. 2021;170:948–63.
Bi H, Wang C, Jiang X, Jiang C, Bao L, Lin Q. Thermodynamics, kinetics, gas emissions and artificial neural network modeling of co-pyrolysis of sewage sludge and peanut shell. Fuel. 2021;284:118988.
Naqvi SR, Tariq R, Hameed Z, Ali I, Taqvi SA, Naqvi M, et al. Pyrolysis of high-ash sewage sludge: thermo-kinetic study using TGA and artificial neural networks. Fuel. 2018;233:529–38.
Buyukada M. Investigation of thermal conversion characteristics and performance evaluation of co-combustion of pine sawdust and lignite coal using TGA, artificial neural network modeling and likelihood method. Bioresour Technol. 2019;287:121461.
Wang S, Shi Z, Jin Y, Zaini IN, Li Y, Tang C, et al. A machine learning model to predict the pyrolytic kinetics of different types of feedstocks. Energy Convers Manag. 2022;260:115613.
Merdun H, Laougé ZB. Kinetic and thermodynamic analyses during co-pyrolysis of greenhouse wastes and coal by TGA. Renew Energy. 2021;163:453–64.
Huang X, Cao JP, Zhao XY, Wang JX, Fan X, Zhao Y, et al. Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel. 2016;169:93–8.
Herce C, De Caprariis B, Stendardo S, Verdone N, De Filippis P. Comparison of global models of sub-bituminous coal devolatilization by means of thermogravimetric analysis. J Therm Anal Calorim. 2014;117:507–16.
Burnham AK, Braun RL. Global kinetic analysis of complex materials. Energy Fuels. 1999;13:1–22.
Lasdon L, Fox R, Ratner M. Nonlinear optimization using the generalized. Inform Reč Opérationn. 1974;3:73–103.
Miura K, Maki T. A simple method for estimating f(E) and k0(E) in the distributed activation energy model. Energy Fuels. 1998;12:864–9.
He Y, Chang C, Li P, Han X, Li H, Fang S, et al. Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis. Bioresour Technol. 2018;259:294–303.
Mishra RK, Mohanty K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol. 2018;251:63–74.
Kaur R, Gera P, Jha MK, Bhaskar T. Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour Technol. 2018;250:422–8.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C. 1964;6:183–95.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polymer letters. 1966;4:323–8.
Hao H, Chang T, Cui L, Sun R, Gao R. Theoretical study on the mechanism of hydrogen donation and transfer for hydrogen-donor solvents during direct coal liquefaction. Catalysts. 2018;8:684.
He Q, Ding L, Gong Y, Li W, Wei J, Yu G. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis. Bioresour Technol. 2019;280:104–11.
Yıldız Z, Uzun H, Ceylan S, Topcu Y. Application of artificial neural networks to co-combustion of hazelnut husk-lignite coal blends. Bioresour Technol. 2016;200:42–7.
Al-Yaari M, Dubdub I. Application of artificial neural networks to predict the catalytic pyrolysis of HDPE using non-isothermal TGA data. Polymers (Basel). 2020;12:1813.
Poli AACM, Cirillo MC. On the use of the normalized mean square error in evaluating dispersion model performance. Atmos Environ A Gen Top. 1993;27:2427–34.
Solomon PR, Serio MA, Suuberg EM. Coal pyrolysis: experiments, kinetic rates and mechanisms. Prog Energy Combust Sci. 1992;18:133–220.
Deshpande G, Solomon P, Serio M. Crosslinking reactions in coal pyrolysis. Conference american chemical society, division of fuel chemistry, Meet Toronto, Canada, 1988:332, 5 Jun 1988.
Yan L, Wang W, Liu Y, Wang M, Li F, Bao W. The roles of low molecular compounds on the light aromatics formation during different rank coal pyrolysis. J Energy Inst. 2022;100:129–36.
Fletcher TH, Kerstein AR, Pugmire RJ, Grant DM. Chemical percolation model for devolatilization. 2. Temperature and heating rate effects on product yields. Energy Fuels. 1990;4:54–60.
Mallick D, Poddar MK, Mahanta P, Moholkar VS. Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour Technol. 2018;261:294–305.
Collard FX, Blin J. A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev. 2014;38:594–608.
Mishra G, Kumar J, Bhaskar T. Kinetic studies on the pyrolysis of pinewood. Bioresour Technol. 2015;182:282–8.
Toloue Farrokh N, Askari M, Fabritius T. Investigation of Tabas anthracite coal devolatilization: kinetics, char structure and major evolved species. Thermochim Acta. 2017;654:74–80.
Acknowledgements
This research was supported by a grant from the National Research Foundation of Korea and funded by the Ministry of Science, ICT, and Future Planning (Grant No. 2022K1A4A8A01080312).
Author information
Authors and Affiliations
Contributions
TVT, TYJ, BHL, and CHJ conceived and planned the research. TVT conducted the experiments. TVT, BHL, and CHJ contributed to the results analysis. TVT and CHJ contributed to the review of the original and revised versions of the manuscript. TVT took the lead in writing the manuscript. All authors helped shape the research, discussed the results, and contributed to the final manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trinh, V.T., Lee, BH., Jeong, TY. et al. Pyrolysis of different rank fuels: characteristics and kinetic parameter study using nonlinear optimization and artificial neural network. J Therm Anal Calorim 148, 5493–5507 (2023). https://doi.org/10.1007/s10973-023-12084-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12084-6