Abstract
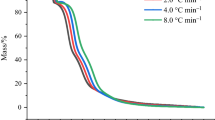
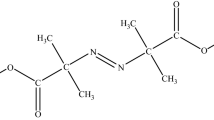
In this work, we used C80 Micro-calorimeter and STA-FTIR-MS instrument to analyze decomposition mechanism of DBAD and evaluated the thermal hazard by the key index on self-accelerating decomposition temperature (SADT). Based on C80 data, the heat release per unit mass of DBAD is 699.85 ± 52.88 kJ kg−1, and the activation energy calculated by Friedman method ranges from 28.58 to 52.03 kJ mol−1. Besides, the thermal decomposition reaction of DBAD can be described by Zhuralev-Lesokin-Tempelman equation. In summary, the decomposition mechanism of DBAD is as follows: the C–O bond cracks firstly, separating ·C(CH3)3 out, then the C–C bond cracks, separating ·CH3 out; the N=N double bond, C–N bond and C–O bond crack subsequently, separating: N–CO, ·O–CO–N:, HO–CO–N: and ·N=N–CO out, and these radical groups gradually decompose or oxidize into ·CH=CHCH3, ·O–CO/CO2, ·COOH, etc.; finally, all these radical groups dissociate and oxidize into H2O, CO2 and N2. The SADT calculated by Semenov model of DBAD under 25 kg standard package is 63.95 °C.
















Similar content being viewed by others
Abbreviations
- TMRad :
-
Time to maximum rate under adiabatic conditions (min)
- TCL :
-
Time to conversion limit (day)
- δH :
-
Enthalpy changes (kJ mol−1)
- α :
-
Reaction progress (ratio of reacted material to total material)
- f(α):
-
Mechanism function
- E a :
-
Activation energy (kJ mol−1)
- A :
-
Pre-exponential factor of Arrhenius equation (s−1)
- β :
-
Heating rate (°C min−1)
- R :
-
Gas constant (J mol−1K−1)
- y(α):
-
Definition function of Malek method
- R 2 :
-
Square value of the correlation coefficient
- T p :
-
Decomposition peak temperature of DBAD (°C)
- T onset :
-
Initial decomposition temperature (°C)
- H p :
-
Maximum heat flow rate (mW)
- ∆H :
-
Heat release per unit mass (kJ kg−1)
- λ :
-
Wavenumber (cm−1)
- c p :
-
Specific heat of reactant (J mol−1K−1)
- M 0 :
-
Reactant mass (g)
- T 0 :
-
Ambient temperature (K)
- U :
-
Surface heat transfer coefficient (J m−2K−1s−1)
- S :
-
Surface area (m2)
- q G :
-
Heat release curve
- q L :
-
Cooling curve
- TNR:
-
Temperature of no return (°C)
References
Bingnan Z. Study on the reaction of azodicarboxylate and sulfur-containing unsaturated double bond system. Ph.D. Thesis; BUCT. 2019; https://doi.org/10.26939/d.cnki.gbhgu.2019.000107.
Mitsunobu O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. In: Stec WJ, editor. Phosphorus chemistry directed towards biology. Pergamon: Elsevier; 1980. p. 213–8.
Hanrahan JR, Mewett KN, Chebib M, et al. Diastereoselective synthesis of (+/-)-(3-aminocyclopentane)alkylphosphinic acids, conformationally restricted analogues of GABA. Org Biomol Chem. 2006;4(13):2642–9. https://doi.org/10.1039/b604002k.
Smith AB, Safonov IG, Corbett RM. Total synthesis of (+)-zampanolide. J Am Chem Soc. 2001;49(123):12426–7. https://doi.org/10.1021/ja012220y.
Kang SB, Ahn EJ, Kim Y, et al. A facile synthesis of (S)-(−)-7,8-difluoro-3,4-dihydro-3-methyl-2H-1,4-benzoxazine by zinc chloride assisted mitsunobu cyclization reaction. Tetrahedron Lett. 1996;37(52):9317–20. https://doi.org/10.1016/S0040-4039(97)82952-8.
Martin SF, Dodge JA. Efficacious modification of the mitsunobu reaction for inversions of sterically hindered secondary alcohols. Tetrahedron Lett. 1991;32(26):3017–20. https://doi.org/10.1016/0040-4039(91)80675-V.
Weissman SA, Rossen K, Reider PJ. Stereoselective synthesis of styrene oxides via a mitsunobu cyclodehydration. Org Lett. 2001;16(3):2513–5. https://doi.org/10.1021/ol016167u.
Pires M. Di-tert-butyl azodicarboxylate. Synlett. 2012;23(11):1071–2. https://doi.org/10.1055/s-0031-1290427.
Winder C, Azzi R, Wagner D. The development of the globally harmonized system (GHS) of classification and labelling of hazardous chemicals. J Hazard Mater. 2005;125(1–3):29–44. https://doi.org/10.1016/j.jhazmat.2005.05.035.
United Nations. Recommendations on the transport of dangerous goods: model regulations. In: 20th ed. United Nations Publications; 2017.
Urben PG. Bretherick's handbook of reactive chemical hazards. In: 7th ed. department of labor occupational health & safety administration: Academic Press; 2006. https://doi.org/10.1016/C2009-1-28334-1.
Qian Y, Shanghao L, Ziru G, et al. Thermal decomposition characteristics of diethyl azodicarboxylate dissolved in three ionic liquids as solvents. J Mol Liq. 2020;302:112564. https://doi.org/10.1016/j.molliq.2020.112564.
Jianmei L, Zhibin Z, Min S. Lewis acid or Brønsted acid catalyzed reactions of vinylidene cyclopropanes with activated carbon-nitrogen, nitrogen-nitrogen, and iodine-nitrogen double-bond-containing compounds. Chem-Eur J. 2009;4(15):963–71. https://doi.org/10.1002/chem.200801785.
Berger A, Wehrstedt KD. Azodicarboxylates: Explosive properties and DSC measurements. J Loss Prevent Proc. 2010;23(6):734–9. https://doi.org/10.1016/j.jlp.2010.06.019.
Chinlung C, Shanghao L, Chenrui C, et al. Multiapproach thermodynamic and kinetic characterization of the thermal hazards of 2,2′-azobis(2-methylpropionate) alone and when mixed with several solvents. J Loss Prevent Proc. 2018;51:150–8. https://doi.org/10.1016/j.jlp.2017.12.003.
Chiafeng T, Ljyh W, Shanghao L, et al. Evaluation of thermal hazard characteristics of four low temperature reactive azo compounds under isothermal conditions. J Loss Prevent Proc. 2021;7:104453. https://doi.org/10.1016/j.jlp.2021.104453.
Minjun P. Study on thermal hazard and thermal decomposition mechanism of two azo compounds. M.S. Thesis; Nanjing University of Science and Technology. 2014; https://doi.org/10.7666/d.Y2520580.
Song G, Wei W, Chen C, et al. Thermal decomposition kinetic evaluation and its thermal hazards prediction of AIBN. J Therm Anal Calorim. 2013;113:1169–76. https://doi.org/10.1007/s10973-013-2993-7.
Jie Z, Ling S, Junying Z. The thermal decomposition mechanism of azodicarbonamide and the effect of zinc oxide on its decomposition. J BUCT Nat Sci Ed. 2011. https://doi.org/10.3969/j.issn.1671-4628.2011.03.008.
Min J, Song G, Shang G, et al. Thermal decomposition mechanism of diisopropyl azodicarboxylate and its thermal hazard assessment. Thermochim Acta. 2020. https://doi.org/10.1016/j.tca.2020.178601.
Ning R, Fang W, Jianjun Z, et al. Progress in thermal analysis kinetics. Acta Phys-Chim Sin. 2020;36(6):12–8. https://doi.org/10.3866/PKU.WHXB201905062.
Jinqiong Z. Solution and analysis of activation energy in thermal analysis. Ph.D. Thesis; BUCT. 2006; https://doi.org/10.7666/d.y1000508.
Šesták J, Málek J. Diagnostic limits of phenomenological models of heterogeneous reactions and thermal analysis kinetics. Solid State Ionics. 1993;63–65:245–54. https://doi.org/10.1016/0167-2738(93)90113-H.
Málek J, Criado JM. A simple method of kinetic model discrimination. Part 1. Analysis of differential non-isothermal data. Thermochim Acta. 1994;236:187–97. https://doi.org/10.1016/0040-6031(94)80267-X.
Zurong H. Thermal analysis kinetics. China: Science Press; 2008.
Zhijie Z. Material physical chemistry. Chemical Industry Press; 2006.
Koga N, Tanaka H. Accommodation of the actual solid-state process in the kinetic model function. J Therm Anal Calorim. 1994;41:455–69. https://doi.org/10.1007/BF02549327.
Koga N, Málek J. Accommodation of the actual solid-state process in the kinetic model function. Part 2. Applicability of the empirical kinetic model function to diffusion-controlled reactions. Thermochim Acta. 1996;69–80:282–3. https://doi.org/10.1016/0040-6031(96)02822-5.
Jinhua S, Shouxiang L, Zhanhui S. Thermal risk assessment method of self-reactive chemical substances. China Safety Sci J. 2003;4:44. https://doi.org/10.3969/j.issn.1003-3033.2003.04.014.
Ruijun F, Xuanjun W, Daizhi L. Research progress on evaluation methods for thermal stability and thermal safety of energetic materials. Chem Prop Polymer Mat. 2004;2(2):22–4. https://doi.org/10.3969/j.issn.1672-2191.2004.02.006.
Boyun L, Xianglie Y, Jinshui Q. Semenov solution and experimental method of thermal ignition critical parameters. J Therm Sci Tech-Jpn. 2013;12(3):255–9. https://doi.org/10.3969/j.issn.1671-8097.2013.03.012.
Regulations on the Safety Management of Hazardous Chemicals, Chemical standards. Metrology. Quality. 2004;05. p. 17–26.
Libing W. Progress of research on the packaging, storage, transportation and management standards of hazardous chemicals. China Petrol Chem Stand Qual. 2006;26(3):34–7. https://doi.org/10.3969/j.issn.1673-4076.2006.03.009.
Funding
This work was supported by National Natural Science Foundation of China, Project 51974166.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, M., Guo, S., Chen, S. et al. Thermal decomposition mechanism and hazard assessment of di-tert-butyl azodicarboxylate (DBAD). J Therm Anal Calorim 148, 4317–4331 (2023). https://doi.org/10.1007/s10973-023-11992-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-11992-x