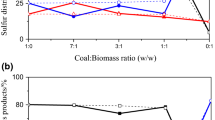
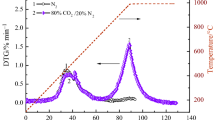
Abstract
In this study, the effects of different co-pyrolysis ways (direct mixed co-pyrolysis and indirect layer-separated co-pyrolysis) on sulfur transformation behavior of Yangquan raw, deashed and depyrited coals were investigated during their co-pyrolysis with biomass (corn-cob) in Ar and CO2 atmospheres. Under the same pyrolysis way, the desulfurization ratios of these coals under CO2 atmosphere are all higher than those under Ar atmosphere, while their char yields lower than those under Ar atmosphere. During the same atmosphere, the char yields of the same kind of coal are almost similar during single pyrolysis and indirect layer-separated co-pyrolysis. However, the char yields (Ymix) during the direct mixed pyrolysis are higher than those (Y3) during the indirect layer-separated co-pyrolysis. Under CO2 atmosphere, the main sulfur-containing gases are composed by H2S and COS, while only H2S in Ar atmosphere, no obvious SO2 emission was detected. During the same co-pyrolysis way, the calorific values of YQ chars under Ar atmosphere are higher than those under CO2 atmosphere. The trend of total release amount of sulfur-containing gases is: indirect layer-separated co-pyrolysis > direct mixed co-pyrolysis > single-pyrolysis. This indicates that H2, CO, CH4 or a small amount of hydrocarbons resulting from biomass decomposition can promote more sulfurs to decompose. But, biomass oil or black carbon from biomass pyrolysis can prohibit the release of sulfur-containing gases. Thus, this study can provide the detailed information about the effects of different co-pyrolysis ways on sulfur transformation of coals under different atmospheres.










Similar content being viewed by others
References
Bai X, Ding H, Lian J, Ma D, Yang X, Sun N, Xue W, Chang Y. Coal production in China: past, present, and future projections. Int Geol Rev. 2017;60:535–47.
Squalli J. Renewable energy, coal as a baseload power source, and greenhouse gas emissions: evidence from U.S. state-level data. Energy. 2017;127:479–88.
Jia Z, Lin B. How to achieve the first step of the carbon-neutrality 2060 target in China: the coal substitution perspective. Energy. 2021;233:0360–5442.
Guo H, Fu Q, Zhang L, Liu F, Hu Y, Zhang H, Hu R. Sulfur K-edge XAS study of sulfur transformation behavior during pyrolysis and co-pyrolysis of biomass and coals under difffferent atmospheres. Fuel. 2018;234:1322–7.
Liu Q, Hu H, Zhou Q, Zhu S, Chen G. Understanding energy efficiency and its drivers: an empirical analysis of China’s 14 coal intensive industries. Energy. 2019;190:0360–5442.
Liu L, Fei J, Cui M, Hu Y, Wang J. XANES spectroscopic study of sulfur transformations during co-pyrolysis of a calcium-rich lignite and a high-sulfur bituminous coal. Fuel Process Technol. 2014;121:56–62.
Melikoglu M. Clean coal technologies: a global to local review for Turkey. Energ Strat Rev. 2018;22:313–9.
Wang C, Liu H, Zhang Y, Zou C, Edward J, Anthony. Review of arsenic behavior during coal combustion: volatilization, transformation, emission and removal technologies. Prog Energy Combust Sci. 2018;68:1–28.
Zhao Y, Xing W, Lu W, Zhang X, Thomas H, Christensen. Environmental impact assessment of the incineration of municipal solid waste with auxiliary coal in China. Waste Manag. 2012;32:1989–98.
Sebastian C, Peter. Reduction of CO2 to chemicals and fuels: a solution to global warming and energy crisis. ACS Energy Lett. 2018;3:1557–61.
Spörl R, Maier J, Scheffffknecht G. Sulphur oxide emissions from dust-fifired oxy-fuel combustion of coal. Energy Procedia. 2013;37:1435–47.
Antar M, Lyu D, Nazari M, Shah A, Zhou X, Smith DL. Biomass for a sustainable bioeconomy: an overview of world biomass production and utilization. Renew Sustain Energy Rev. 2021;139:1364–321.
Toklu E. Biomass energy potential and utilization in Turkey. Renew Energy. 2017;107:235–44.
Haykiri-Acma H, Yaman S. Interaction between biomass and different rank coals during co-pyrolysis. Renew Energy. 2010;35:288–92.
Wu Z, Yang W, Li Y, Yang B. Co-pyrolysis behavior of microalgae biomass and low-quality coal: products distributions, char-surface morphology, and synergistic effects. Biores Technol. 2018;255:238–45.
Wang X, Guo H, Liu F, Hu R, Wang M. Effects of CO2 on sulfur removal and its release behavior during coal pyrolysis. Fuel. 2016;165:484–9.
Yang N, Guo H, Liu F, Zhang H, Hu Y, Hu R. Effects of atmospheres on sulfur release and its transformation behavior during coal thermolysis. Fuel. 2018;215:446–53.
Wang B, Zhao S, Huang Y, Zhang J. Effect of some natural minerals on transformation behavior of sulfur during pyrolysis of coal and biomass. J Anal Appl Pyrol. 2014;105:284–94.
Masnadi M, Habibi R, Kopyscinski J, Hill J, Bi X, Jim Lim C, Ellis N, Grace J. Fuel characterization and co-pyrolysis kinetics of biomass and fossil fuels. Fuel. 2014;117:1204–14.
Salema AA, Ting RMW, Shang YK. Pyrolysis of blend (oil palm biomass and sawdust) biomass using TG-MS. Bioresour Technol. 2019;274:439–46.
Qin Y, Han Q, Zhao Z, Du Z, Feng J, Li W, Vassilev S, Vassileva C. Impact of biomass addition on organic structure and mineral matter of char during coal-biomass co-gasifification under CO2 atmosphere. Fuel. 2017;202:556–62.
Idris S, Rahman N, Ismail K, Alias A, Rashid Z, Aris M. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Biores Technol. 2010;101:4584–92.
Moghtaderi B, Meesri C, Wall T. Pyrolytic characteristics of blended coal and woody biomass. Fuel. 2004;83:745–50.
Corderoa T, Rodrı´guez-Mirasola J, Pastranaa J, Rodrı´guezb J. Improved solid fuels from co-pyrolysis of a high-sulphur content coal and different lignocellulosic wastes. Fuel. 2004;83:1585–90.
Liu L, Liu H, Cui M, Hu Y, Wang J. Calcium-promoted catalytic activity of potassium carbonate for steam gasification of coal char: transformation of sulfur. Fuel. 2013;112:687–94.
Zhang J, Wang M, Chen W, et al. Effects of step acid treatment process on the structure and pyrolysis characteristics of Ximeng brown coal: formation of gaseous products. J Fuel Chem Technol. 2013;41(10):1160–5.
Accolla FV, Orr WL. Pyrite removal from Kerogen without altering organic matter: the chromous chloride method. Energy Fuel. 1993;7(3):406–10.
Shirazi AR, Börtin O, Eklund L, Lindqvist O. The impact of mineral matter in coal on its combustion, and a new approach to the determination of the calorific value of coal. Fuel. 1995;74:247–51.
Mason DM, Gandhi KN. Formulas for calculating the calorific value of coal and coal chars: development, tests, and uses. Fuel. 1983;7:11–2.
Acknowledgements
This study was financially supported by the Project of Natural Science Foundation of China (No.21865018) and the Project of Natural Science Foundation of Inner Mongolia (No. 2019MS02001).
Author information
Authors and Affiliations
Contributions
XH performed the experimental results, data curation, writing—original draft. YL contributed to data curation and formal analysis. HG was involved in formal analysis, editing, and funding acquisition. HL contributed to formal analysis. FL was involved in formal analysis, funding acquisition, resources, supervision, writing—original draft, and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, X., Guo, H., Liu, H. et al. Effects of different co-pyrolysis ways on sulfur transformation of coals under different atmospheres. J Therm Anal Calorim 148, 4345–4358 (2023). https://doi.org/10.1007/s10973-023-11958-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-11958-z