Abstract
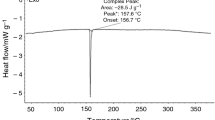
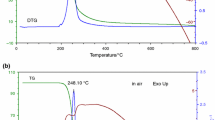
In this work, thermal decomposition processes of (R)-(–)-phenylephrine hydrochloride (R-PEHC) in nitrogen (N2) and air atmosphere were investigated by multiple scanning rate method ranged from 300 to 950 K under 0.1 MPa. Five kinds of heating rates including 5 K min−1, 10 K min−1, 15 K min−1, 20 K min−1 and 25 K min−1 were applied during determination process of thermogravimetric differential scanning calorimetry (TG-DSC) of R-PEHC. Thermal decomposition processes were analyzed comprehensively according to TG-DSC results, bond length and bond energy data. Results indicated that thermal decomposition process of R-PEHC in N2 could be divided into three stages according to breaking of chemical bond, while thermal decomposition process in air was more complex. The similarity between two kinds of decomposition processes was that the first stage was the fractures of C···C and C···N on straight chain; meanwhile, first stage was considered as active decomposition stage. Given the importance of first stage of thermal decomposition process, corresponding average activation energies were calculated by Flynn–Wall–Ozawa and Starink method. Besides, based on obtained activation energy values, the pre-exponential factor and kinetic equation of thermal decomposition process were obtained by Málek method. Furthermore, based on results of kinetics parameters, theoretical storage period of R-PEHC in selected atmospheres (N2, air) and thermodynamic parameters of first stage of thermal decomposition process were also investigated. The results suggested that storage periods of R-PEHC in selected atmospheres were not less than three years at 298.15 K; meanwhile, thermal decomposition processes in selected atmospheres were always endothermic, non-spontaneous and entropy reduction process.

















Similar content being viewed by others
References
Wang Y, Chen WJ, Zhou Y, Huang M, He WY, Yao QQ, Zhang QM, Deng YL, Zhang YK. Chiral separations of adrenaline and phenylephrine hydrochloride by cellulose ramification chiral stationary phases. Chin J Pharm Anal. 2012;32:1985–90.
Liu CH, Sun QY, Li X, Zhang GQ, Wang B, Chai YF. Research of separation of enantiomers of adrenalines by capillary zone electrophoresis. Chin J Anal Lab. 2007;26(7):64–6. https://doi.org/10.3969/j.issn.1000-0720.2007.07.017.
Ni G. Control of the major degradation product of Phenylephrine HCl in the compound table. Chin Master Theses. 2017. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1016273040.nh&DbName=CMFD2017
Pan T, Cheng YW, Wang LJ, Li X. Thermal decomposition kinetics of ammonium sulfate studied with multiple scanning methods. J Chem Eng Chin Univ. 2019;33(05):1086–91.
Hazardous Substances Data Bank (HSDB). USA: National Library of Medicine; 2004.
Tournier RF. Presence of intrinsic growth nuclei in overheated and undercooled liquid elements. Phys B. 2007;392(1–2):79–91. https://doi.org/10.1016/j.physb.2006.11.002.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77(18):3865–8. https://doi.org/10.1016/physrevlett.77.3865.
Grimme S. Density functional theory with London dispersion corrections. Wiley Interdiscip Rev: Comput Mol Sci. 2011;1(2):211–28. https://doi.org/10.1002/wcms.30.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci, Part B. 1966;4(5):323–8. https://doi.org/10.1002/pol.1966.110040504.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. https://doi.org/10.1246/bcsj.38.1881.
Parajó JJ, Teijeira T, Fernández J, Salgado J, Villanueva M. Thermal stability of some imidazolium [NTf2] ionic liquids: isothermal and dynamic kinetic study through thermogravimetric procedures. J Chem Thermodyn. 2017;112:105–13. https://doi.org/10.1016/j.jct.2017.04.016.
Muraleedharan K. Thermal decomposition kinetics of potassium iodate part I the effect of particle size on the rate and kinetics of decomposition. J Therm Anal Calorim. 2012;109:237–45. https://doi.org/10.1007/s10973-011-1711-6.
Chen FX, Zhou CR, Li GP. Study on thermal decomposition and the non-isothermal decomposition kinetics of glyphosate. J Therm Anal Calorim. 2012;109:1457–62. https://doi.org/10.1007/s10973-011-1834-9.
Doyle CD. Series approximations to the equation of thermogravimetric data. Nature. 1965;207:290–1. https://doi.org/10.1038/207290a0.
Starink MJ. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim Acta. 1996;288(1–2):97–104. https://doi.org/10.1016/S0040-6031(96)03053-5.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of iso-conversion methods. Thermochim Acta. 2003;404(1–2):163–76. https://doi.org/10.1016/S0040-6031(03)00144-8.
Tian Y, Zhao DF, Shu CM, Roy N, Qi M, Liu Y. Study on thermal stability and thermal decomposition mechanism of 1-((cyano-1-methylethyl) azo) formamide. Process Saf Environ Prot. 2021;155:219–29. https://doi.org/10.1016/j.psep.2021.09.018.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69. https://doi.org/10.1016/0040-6031(92)85118-F.
Málek J. A computer program for kinetic analysis of non-isothermal thermoanalytical data. Thermochim Acta. 1989;138(2):337–46. https://doi.org/10.1016/0040-6031(89)87270-3.
Munir S, Daood SS, Nimmo W, Cunliffe AM, Gibbs BM. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresour Technol. 2009;100(3):1413–8. https://doi.org/10.1016/j.biortech.2008.07.065.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal Calorim. 1977;11:445–7. https://doi.org/10.1007/BF01903696.
Zhang WL, Zhang J, Ding YM, He QZ, Lu KH, Chen HY. Pyrolysis kinetics and reaction mechanism of expandable polystyrene by multiple kinetics methods. J Cleaner Prod. 2021;285:125042. https://doi.org/10.1016/j.jclepro.2020.125042.
Sakhiya AK, Anand A, Vijay VK, Kaushal P. Thermal decomposition of rice straw from rice basin of India to improve energy-pollution nexus: kinetic modeling and thermodynamic analysis. Energy Nexus. 2021;4:100026. https://doi.org/10.1016/j.nexus.2021.100026.
Sronsri C, Noisong P, Danvirutai C. Thermal decomposition kinetics of Mn0.9Co0.1HPO4·3H2O using experimental-model comparative and thermodynamic studies. J Therm Anal Calorim. 2017;127:1983–94. https://doi.org/10.1007/s10973-016-5720-3.
Xu FY, Wu XL, Wang X, Luo YM, Ma T, Liu DB, Xu S. Kinetic characteristics of thermal decomposition and thermal safety for methylhydrazine. Chin J Energ Mater. 2022;30(2):171–7.
Yuan JJ, Ye JZ, Wang CZ, Liu YH. Thermal stability and decomposition kinetics of hydroxytyrosol. Chem Ind For Prod. 2016;36(6):87–92. https://doi.org/10.3969/j.issn.0253-2417.2016.06.014.
Xu F. Calorimetry and thermal analysis studies on thermodynamic properties of drugs, Chin Doct Diss. 2005. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFD9908&filename=2005104711.nh
Bhaduri D, Saha NN. Structure of an adrenergic drug: L-phenylephrine hydrochloride, C9H14NO2+Cl-. Acta Crystallogr Sect C: Struct Chem. 1982;39:350–3. https://doi.org/10.1107/S0108270183004680.
Tao YP, Han LG. Raman spectroscopy and normal vibration analysis of phenol. J Luoyang Norm Uni. 2013;32(05):28–31.
Mahadevan D, Periandy S, Ramalingam S. Vibrational spectroscopy (FT-IR and FT-Raman) investigation using ab initio (HF) and DFT (B3LYP) calculations on the structure of 3-Bromo phenol. Spectrochim Acta, Part A. 2011;78:575–81. https://doi.org/10.1016/j.saa.2014.08.013.
Sundaraganesan N, Saleem H, Mohan S, Ramalingam M. FT-Raman and FTIR spectra, assignments and ab initio calculations of 2-aminobenzyl alcohol. Spectrochim Acta, Part A. 2005;61:377–85. https://doi.org/10.1016/j.saa.2004.04.012.
Wong SP, Xu YZ. Fourier transform infrared spectroscopy. 3rd ed. Beijing: Chemical Industry Press; 2016.
Wan YM, Zhao R, Zhang PS, He HX, Sha J, Li T, Ren BZ. Solid-liquid equilibrium solubility, thermodynamic properties, solvent effect and molecular simulation of (R)-(-)-phenylephrine hydrochloride in ten pure solvents ranged from 278.15 K to 323.15 K. J Chem Thermodyn. 2021;144:105959. https://doi.org/10.1016/j.jct.2019.105959.
Milne GWA. Drugs: synonyms & properties. 2nd ed. London: Brookfied VT; 2002.
Botha SA, Lotter AP, Preez JLD. DSC screening for drug-drug interactions in polypharmaceutials intended for the alleviation of the symptoms of colds and flu II. Drug Dev Ind Pharm. 1987;13(2):345–54. https://doi.org/10.3109/03639048709040177.
Thomas E, Rubino J. Solubility, melting point and salting-out relationships in a group of secondary amine hydrochloride salts. Int J Pharm. 1996;130(2):179–83. https://doi.org/10.1016/0378-5173(95)04269-5.
Luo Y. Handbook of chemical bond energy data. 1st ed. Beijing: Science Press; 2005.
Zhang TT. Pyrolysis Simulations of Various Lignin Molecular Models by ReaxFF Molecular Dynamics. Chin Doct Diss. 2020. https://doi.org/10.27550/d.cnki.ghgys.2020.000003
Yu S. Study on the Process of Anti-solvent Crystallization of Cefathiamidine. Chin Doct Diss. 2019. https://doi.org/10.27151/d.cnki.ghnlu.2019.004140
Wan YM, He HX, Gao XQ, Guo XX, Li FF, Li YX. Solid-liquid equilibrium of 2,3-dimethoxybenzoic acid in fifteen mono-solvents: determination, correlation, Hansen solubility parameter, molecular dynamic simulation and thermodynamic analysis. J Mol Liq. 2020;348:118029. https://doi.org/10.1016/j.molliq.2021.118029.
Liu H, Wang YL, Li Y, Zheng ZX, Huang X, Wang T, Hao HX. Thermodynamic mechanism of stability and polymorphic transformation behaviors of enantiotropic polymorphs of glycolide. J Chem Thermodyn. 2020;149:106145. https://doi.org/10.1016/j.jct.2020.106145.
Li JL, Ji X, Li C, Li ZQ, Wu D, Zhang B, Hou BH, Zhou LN, Xie C, Gong JB, Chen W. Solubility measurement and thermodynamic correlation of (2,4-dichlorophenoxy)acetic acid in fifteen pure solvents. J Chem Thermodyn. 2021;163:106589. https://doi.org/10.1016/j.jct.2021.106589.
Li ZX, Ma YM, Lin JW, Shi P, Wu SG, Han DD, Gong JB. Thermodynamic analysis and molecular dynamic simulation of the solubility of 2,2-Bis(hydroxymethyl)propionic acid in 12 mono-solvents. J Chem Thermodyn. 2022;164:106625. https://doi.org/10.1016/j.jct.2021.106625.
Sronsri CC, Noisong P, Danviruta C. Thermal decomposition kinetics of Mn0.9Co0.1HPO4·3H2O using experimental-model comparative and thermodynamic studies. J Therm Anal Calorim. 2017;127:1983–94. https://doi.org/10.1007/s10973-016-5720-3.
Noisong P, Danvirutai C. Kinetics and mechanism of thermal dehydration of KMnPO4·H2O in nitrogen atmosphere. Ind Eng Chem Res. 2010;49(7):3146–51. https://doi.org/10.1021/ie900993f.
Acknowledgments
This work was financially supported by the Science and technology project of Henan province (No.222102230104). At the same time, we thank the editors and the reviewers for their useful feedback that improved this paper.
Funding
Henan Provincial Science and Technology Research Project, 222102230104, Yameng Wan; Doctoral cultivation fund projects of Henan University of Engineering in 2022 (Investigation on design and synergistic mechanism of aqueous stable large Stokes shift MOF co-crystal fluorescent dyes), Yameng Wan.
Author information
Authors and Affiliations
Contributions
YW took part in conceptualization; methodology; software; validation; formal analysis; investigation; data curation; writing—original draft; software; supervision. HH involved in validation; resources; formal analysis. FL took part in validation; resources; formal analysis. PZ took part in validation; formal analysis. XG took part in validation; formal analysis. YW took part in data curation, validation. ZG involved in validation; software. YL involved in validation; software.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, Y., He, H., Li, F. et al. Thermal stability, thermodynamics and kinetic study of (R)-(–)-phenylephrine hydrochloride in nitrogen and air environments. J Therm Anal Calorim 148, 2483–2499 (2023). https://doi.org/10.1007/s10973-022-11911-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11911-6