Abstract
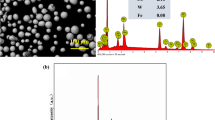
Amorphous nickel-phosphorus alloy as a metallic glass layer can not only protect the substrate from mechanical stress and corrosion but also improve the optical performance. The morphology, structure and chemical composition were studied in the work by using x-ray diffraction (XRD), high resolution transmission electron microscope, scanning electron microscope and in situ energy-dispersive x-ray spectroscopy. Non-isothermal crystallization kinetics in amorphous electroless nickel-phosphorus alloy plating was characterized by differential scanning calorimetry (DSC). The mass percentage concentration of P in the coating surface was about 12.36mass%, which belongs to high phosphorus metal glass coating. All the overall DSC curves with various heating rates have just one single exothermic peak of crystallization. The apparent activation energy E of characteristic temperatures were calculated by using Kissinger and Ozawa approach. Additionally, the local activation energy E(x) was measured by Kissinger–Akahira–Sunose and Ozawa-Flynn-Wall methods demonstrates a progressive declining trend as the crystallization process proceeds, which indicated the crystallization process becomes easier with the increase in temperature. Local activation energy E(x) decreased as the crystal volume fraction (x) increased in the crystallization process. The local Avrami exponents n(x) was estimated by the Johnson–Mehl–Avrami equation, and the values exceed 2.5 at different heating rates, which reflected the crystallization process is a representative three-dimensional diffusion-controlled growth mechanism in the overall crystallization process, and the nucleation rate increased with the increase in the crystallized volume fraction (x). Final results will provide comprehensive insights into the understanding about Ni–P plating formation.










Similar content being viewed by others
References
Brenner A, Riddell GE. Nickel plating on steel by chemical reduction. J Res Nat Bur Stand. 1946;37:31–4.
Lee CK. Structure, electrochemical and wear-corrosion properties of electroless nickel-phosphorus deposition on CFRP composites. Mater Chem Phys. 2009;114:125–33.
Vojtech D, Novák M, Zelinková M, Novák P, Michalcová A, Fabián T. Structural evolution of electroless Ni-P coating on Al-12 wt% Si alloy during heat treatment at high temperatures. Appl Surf Sci. 2009;255:3745–51.
Crobu M, Scorciapino A, Elsener B, Rossi A. The corrosion resistance of electroless deposited nano-crystalline Ni-P alloys. Electrochim Acta. 2008;53:3364–70.
Xu XQ, Mao J, Bai ZQ, Feng YR, Ma QR, Zhang ZW. The corrosion behaviour of electroless Ni-P coating in Cl- /H2S environment. Appl Surf Sci. 2012;258:8802–6.
Sun C, Li JK, Fattahpour V, Roostaeib M, Mahmoudi M, Zeng HB, Luo JL. Insights into the erosion-enhanced corrosion on electroless Ni-P coating from single particle impingement. Corros Sci. 2020;166: 108422.
Ashassi-Sorkhabi H, Eshaghi M. Corrosion resistance enhancement of electroless Ni-P coating by incorporation of ultrasonically dispersed diamond nanoparticles. Corros Sci. 2013;77:185–93.
Magesh G, Elansezhian R. Synthesis and novel development of electroless Ni-P coating on bamboo fibre. Mater Taday. 2020;9:1–6.
Rabizadeh T, Allahkaram SR, Zarebidaki A. An investigation on effects of heat treatment on corrosion properties of Ni-P electroless nano-coatings. Mater Design. 2010;31:3174–9.
Sun C, Zeng HB, Luo JL. Unraveling the effects of CO2 and H2S on the corrosion behavior of electroless Ni-P coating in CO2/H2S/Cl– environments at high temperature and high pressure. Corros Sci. 2019;148:317–30.
Ghavidel N, Allahkaram SR, Naderi R, Barzegar M, Bakhshandeh H. Corrosion and wear behavior of an electroless Ni-P/nano-SiC coating on AZ31 Mg alloy obtained through environmentally-friendly conversion coating. Surf Coat Tech. 2020;382: 125156.
Li J, Shao Z, Zhang X, Tian Y. The electroless nickel-plating on magnesium alloy using NiSO4·6H2O as the main salt. Surf Coat Tech. 2006;200:3010–5.
Li LB, An MZ. Electroless nickel-phosphorus plating on SiCp/Al composite from acid bath with nickel activation. J Alloy Compd. 2008;461:85–91.
Touri S, Monirvaghefi SM. Fabrication and characterization of functionally graded Ni-P electroless coating with variable properties along the surface of the coating. Mater Today Commun. 2020;24:101203–15.
Azli NNA, Mohd Amin NF, Oluhende ST, Mohamad SNA, Fadil NA. Electroless deposited black nickel-phosphorous solar absorber coatings on carbon steel: effect of plating bath pH. Mater Taday. 2021;39:1071–6.
Narayanan TSNS, Baskaran I, Krishnaveni K, Parthiban S. Deposition of electroless Ni-P graded coatings and evaluation of their corrosion resistance. Surf Coat Tech. 2006;200:3438–45.
Gutierrez AGG, Pech-Canul MA, Sebastian PJ. Zincating effect on corrosion resistance of electroless Ni-P coating on aluminum alloy 6061. Fuel Cells. 2017;177:770–7.
Gong P, Yao KF, Zhao SF. Cu-alloying effect on crystallization kinetics of Ti41Zr25Be28Fe6 bulk metallic glass. J Therm Anal Calorim. 2015;121:697–704.
Hu CC, Bai A. Influences of the phosphorous content on physicochemical properties of nickel phosphorous deposits. Mater Chem Phys. 2003;80:215–8.
Liu YY, Yu J, Huang H, Xu BH, Liu XL, Gao Y, Dong XL. Synthesis and tribological behavior of electroless Ni-P-WC nanocomposite coatings. Surf Coat Tech. 2007;201:7246–51.
Minouei H, Akbabi GH, Enayati MH, Hong SI. Non-isothermal nano-crystallization kinetics in amorphous Ni55Nb35Si10 alloy. T Nonferr Metal Soc. 2019;29:358–64.
Bagley BG, Turnbull D. The preparation and crystallization behavior of amorphous nickel-phosphorus thin films. Acta Metal. 1970;18:857–62.
Mahoney MW, Dynes PJ. The effects of thermal history and phosphorus level on the crystallization behavior of electroless nickel. Scripta Metall. 1985;19:539–42.
Keong KG, Sha W, Malinov S. Computer modelling of the non-isothermal crystallization kinetics of electroless nickel-phosphorus deposits. J Non-Cryst Solids. 2003;324:230–41.
Ozawa T. Temperature control modes in thermal analysis. Pure Appl Chem. 2000;72:2083–99.
Keong KG, Sha W, Malinov S. Crystallisation kinetics and phase transformation behaviour of electroless nickel–phosphorus deposits with high phosphorus content. J Alloys Compd. 2002;334:192–9.
Hsiao A, McHenry ME, Laughlin DE, Kramer MJ, Ashe C, Ohkubo T. The thermal, magnetic, and structural characterization of the crystallization kinetics of Fe88Zr7B4Cu1, an amorphous soft magnetic ribbon. IEEE Trans Magn. 2002;38:3039–44.
Gao JQ, Wu YT, Liu L, Shen B, Hu WB. Crystallization temperature of amorphous electroless nickel–phosphorus alloys. Mater Lett. 2005;59:1665–9.
Musahwar N, Majeed Khan MA, Husain M, Zulfequar M. Dielectric and electrical properties of Se-S glassy alloys. Physica B. 2007;396:81–6.
Musahwar N, Khan W, Husain M, Zulfequar M, Majeed Khan MA. Non-isothermal kinetic analysis on the crystallization process in Se-S glassy system. J Therm Anal Calorim. 2012;110:823–9.
Starink MJ. The analysis of Al-based alloys by calorimetry: quantitative analysis of reactions and reaction kinetics. Int Mater Rev. 2004;49:191–226.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Mehta N, Kumar A. Some new observations on activation energy of crystal growth for thermally activated crystallization, mehta and kumar. J Phys Chem B. 2016;120:1175–82.
Sridharan K, Sheppard K. Crystallization of amorphous iron-nickel-phosphorus alloys prepared by electrodeposition. J Mater Process Tech. 1997;68:109–16.
Yazdi SS, Ashrafizadeh F, Hakimizad A. Improving the grain structure and adhesion of Ni-P coating to 3004 aluminum substrate by nanostructured anodic film inter layer. Surf Coat Tech. 2013;232:561–6.
Yin ZW, Chen FY. Effect of nickel immersion pretreatment on the corrosion performance of electroless deposited Ni-P alloys on aluminum. Surf Coat Tech. 2013;228:34–40.
Cui CY, Dua H, Liu HS, Xiong TY. Corrosion behavior of the electroless Ni-P coating on the pore walls of the lotus-type porous copper. Corros Sci. 2020;162:108202–16.
Geng PH, Qin GL, Zhou J. A computational modeling of fully friction contact-interaction in linear friction welding of Ni-based superalloys. Mater Design. 2020;185: 108244.
Ashassi-Sorkhabi H, Rafizadeh SH. Effect of coating time and heat treatment on structures and corrosion characteristics of electroless Ni-P alloy deposits. Surf Coat Tech. 2004;176:318–26.
Lin CJ, Chen KC, He JL. The cavitation erosion behavior of electroless Ni-P-SiC composite coating. Wear. 2006;261:1390–6.
Mehta N. Characterization techniques for the study of thermally activated phase transitions and determination of thermo-physical/kinetic properties. In: Pekar L, editor. Advanced analytic and control techniques for thermal systems with heat exchangers. Elsevier; 2020. p. 149–66.
Karthikeyan S, Vijayaraghavan L, Madhavan S, Almeida A. Study on the mechanical properties of heat-treated electroless NiP coatings reinforced with Al2O3 nano particles. Metall Mater Trans A. 2016;47:2223–31.
Barzegar M, Allahkaram SR, Naderi R, Ghavidel N. Effect of phosphorous content and heat-treatment on the structure, hardness and wear behavior of Co-P coating. Wear. 2019;422–423:35–43.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. Res Natl Bur Stand. 1956;57:217–21.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Afify N. A new method to study the crystallization or chemical kinetics using thermal analysis technique. J Phy Chem Solids. 2008;69:1691–7.
Wellen RMR, Canedo EL. On the kissinger equation and the estimate of activation energies for non-isothermal cold crystallization of PET. Polym Test. 2014;40:33–8.
Kong LH, Gao YL, Song TT, Wang G, Zhai QJ. Non-isothermal crystallization kinetics of FeZrB amorphous alloy. Thermochim Acta. 2011;522:166–72.
Ozawa T. A new method of analyzing thermogravimetric data. J Bull Chem Soc Jpn. 1965;38:1881–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Wang HR, Gao YL, Min GH, Hui XD, Ye YF. Primary crystallization in rapidly solidified Zr70Cu20Ni10 alloy from a supercooled liquid region. Phys Lett A. 2003;314:81–7.
Xu T, Jian ZY, Chang FE, Zhuo LC, Zhang T. Non-isothermal crystallization kinetics of Fe75Cr5P9B4C7 metallic glass with a combination of desired merits. Vacuum. 2018;152:8–14.
Srivastava A, Chandel N, Mehta N. Iso-conversional kinetic analysis of quaternary glass re-crystallization. Heliyon. 2017;3: e00249.
Arun-Pratap ATP. Kinetics of crystallization of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim. 2012;107:159–65.
Maldonado YG, Castillejos EAH. A new method for estimating the isothermal devitrification and crystallization of mold powder slags from non-isothermal DSC data. Mater Design. 2015;83:728–35.
Sommereyns A, Hupfeld T, Gann S, Wang T, Wu CC, Zhuravlev E, Lüddecke A, Baumann S, Rudloff J, Lang M, Gökce B, Barcikowski S, Schmidt M. Influence of sub-monolayer quantities of carbon nanoparticles on the melting and crystallization behavior of polyamide 12 powders for additive manufacturing. Mater Design. 2021;21: 109487.
Peng ZL, Zhou TS, Li YN, Cui Z, Wu ZY, Yan JC. Microstructure and mechanical performance of AZ31/2024 dissimilaralloy joints using a multi-interlayer of Ni/Al/Zn via ultrasonic-assistedtransient liquid phase bonding. Mater Design. 2021;197: 109218.
Wang YX, Guan LL, He Z, Zhang SP, Singh H, Hayat MD, Yao CZ. Influence of pretreatments on physicochemical properties of Ni-P coatings electrodeposited on aluminum alloy. Mater Design. 2021;197: 109233.
Málek J. The applicability of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim Acta. 1995;267:61–73.
Wang Y, Xu K, Li Q. Comparative study of non-isothermal crystallization kinetics between Fe80P13C7 bulk metallic glass and melt-spun glassy ribbon. J Alloy Compd. 2012;540:6–15.
Ke HB, Xu HY, Huang HG, Liu TW, Zhang P, Wu M, Zhang PG, Wang YM. Non-isothermal crystallization behavior of U-based amorphous alloy. J Alloy Compd. 2017;691:436–41.
Wu JL, Pan Y, Pi JH. On non-isothermal kinetics of two Cu-based bulk metallic glasses. J Therm Anal Calorim. 2013;115:267–74.
Lu W, Yan B, Huang WH. Complex primary crystallization kinetics of amorphous Finemet alloy. J Non-Cryst Solids. 2005;351:3320–4.
Bianchi O, Oliveira RVB, Fiorio R, Martins JDN, Zattera AJ, Canto LB. Assessment of Avrami, Ozawa and Avrami-Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polymer Test. 2008;27:722–9.
Ranganathan S, Von Heimendahl M. The three activation energies with isothermal transformations: applications to metallic glasses. J Mater Sci. 1981;16:2401–4.
Hyun JI, Kim CI, Nam SW, Kim WT, Kim DH. Nanoscale phase separation and microstructure evolution during crystallization in Al-Si-Ni amorphous alloy. Mater Design. 2020;192: 108719.
León-Patiñoa CA, García-Guerra J, Aguilar-Reyes EA. Tribological characterization of heat-treated Ni-P and Ni-P-Al2O3 composite coatings by reciprecating sliding tests. Wear. 2019;426–427:330–40.
Perejon A, Sanchez-Jimenez PE, Criado JM, PerezMaqueda LA. Kinetic analysis of complex solid-state reactions: a new deconvolution procedure. J Phys Chem B. 2011;115:1780–91.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number: 51971166). The Natural Science Basic Research Program of Shaanxi (Grant Number: 2020JQ-811)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, M., Jian, Z., Hai, R. et al. Non-isothermal crystallization kinetics of amorphous electroless nickel-phosphorus alloy plating. J Therm Anal Calorim 148, 1959–1970 (2023). https://doi.org/10.1007/s10973-022-11828-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11828-0