Abstract
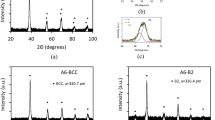
In this paper, the crystallization kinetics of Hf26Be18Ti18Zr18Cu7.5Ni12.5 high mixing entropy amorphous alloys under non-isothermal conditions are studied. The alloy shows two different crystallization events. In addition, the activation energies of the two crystallization events are calculated using the Kissinger, Augis-Bennett, and Ozawa methodologies. Similar values are obtained by the three equations. The activation energy of the first crystallization event is slightly less than that of the second crystallization events, which indicates that the first crystallization can easily occur. The modified Johnson–Mehl–Avrami (JMA) equation is then used to further analyze the non-isothermal crystallization kinetics. The Avrami exponent (n(α)) is between 1.5 and 2.5 for the first crystallization even and most instances (0.1 < α < 0.5) of the second crystallization event, which demonstrates that the crystallization mechanism has mainly been controlled by a three-dimensional growth with a nucleation rate decrease. Moreover, n(α) is between 1 and 1.5 in the second stage of the second crystallization event (0.5 < α < 0.9), which implies a direct growth of crystal nuclei. Compared with the other alloys, Hf26Be18Ti18Zr18Cu7.5Ni12.5 has stronger high entropy effect, leading to more sluggish diffusion and more difficult crystallization.









Similar content being viewed by others
References
Inoue A, Wang XM, Zhang W. Developments and applications of bulk metallic glasses. Rev Adv Mater Sci. 2008;18:1–9.
Inoue A. High strength bulk amorphous alloys with low critical cooling rates(Overview). Mater Trans JIM. 1995;36(7):866–75.
Inoue A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000;48:279–306.
Inoue A, Zhang T, Saida J, Matsushita M, Chen MW, Sakurai T. High strength and good ductility of bulk quasicrystalline base alloys in Zr65Al7.5Ni10Cu7.5-xPdx system. Mater Trans JIM. 1999;40(10):1137–43.
Shcheretskyy OA, Lakhnenko VL, Shumikhin VS, Bespalyy AA, Soloviova AV. Fabrication of nanostructural materials by means of heattreatment of amorphous Zr64Cu16Ni10Al9.5Nb0.5 alloy Metallofiz. Noveishie Tekhnol. 2011;33(10):1323–32.
Pelletier JM, Louzguine-Luzgin DV, Li S, Inoue A. Elastic and viscoelastic properties of glassy quasicrystalline and crystalline phases in Zr65Cu5Ni10Al7.5Pd12.5 alloys. Acta Mater. 2011;59:2797–806.
Sun BR, Xin SW, Shen TD. Low-temperature magnetization and magnetic exchange interactions in Fe40Ni40P14B6 bulk metallic glasses. J Magn Magn Mater. 2017;429:276–80.
Aronhime N, DeGeorge V, Keylin V, Ohodnicki P, McHenry ME. The effects of strain-annealing on tuning permeability and lowering losses in Fe-Ni-based metal amorphous nanocomposites. JOM. 2017;69:2164–70.
Takeuchi A, Chen N, Wada T, Yokoyama Y, Kato H, Inoue A. Pd20Pt20Cu20Ni20P20 high-entropy alloy as a bulk metallic glass in the centimeter. Intermetallics. 2011;19:1546–54.
Gong P, Yao KF, Ding HY. Crystallization kinetics of HfTiZrCuNiBe high entropy bulk metallic glass. Mater Lett. 2015;156:146–9.
Zhao SF, Shao Y, Liu X, Chen N, Ding HY, Yao KF. Pseudoquinary Ti20Zr20Hf20Be20(Cu20-xNix) high entropy bulk metallic glasses with large glass forming ability. Mater Des. 2015;87:625–31.
Cao QP, Liu JW, Li JF, Zhou YH, Wang XD, Jiang JZ. Isochronal crystallization kinetics of Cu60Zr20Ti20 bulk metallic glass. J Non Cryst Solids. 2011;357:1182–7.
Li B, Li Yh, Yang K, Li JS, Fan XH. Effect of yttrium addition on the non-isothermal crystallization kinetics and fragility of Cu– Zr–Al bulk metallic glass. Thermochim Acta. 2016;642:105–10.
Wang XF, Wang D, Zhu B, Li YJ, Han FS. Crystallization kinetics and thermal stability of mechanically alloyed Al76Ni8Ti8Zr4Y4 glassy powder. J Non Cryst Solids. 2014;385:111–6.
Cui J, Li JS, Wang J, Kou HC, Qiao JC, Gravierc S, Blandinc JJ. Crystallization kinetics of Cu38Zr46Ag8Al8 bulk metallic glass in different heating conditions. J Non Cryst Solids. 2014;404:7–12.
Hu XX, Jichao Q, Pelletier JM, Yao Y. Evaluation of thermal stability and isochronal crystallization kinetics in the Ti40Zr25Ni8Cu9Be18 bulk metallic glass. J Non Cryst Solids. 2016;432:254–64.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modifification of the Kissinger method. J Therm Anal Calorim. 1978;13:283–99.
Gong P, Li FW, Yin G, Deng L, Wang XY, Jin JS. Thermal cycling effect on the kinetics of glass transition and crystallization of a Zr-based bulk metallic glass. J Therm Anal Calorim. 2020;142:63–73.
Zhuang YX, Duan TF, Shi HY. Calorimetric study of nonisothermal crystallization kinetics of Zr60Cu20Al10Ni10 bulk metallic glass. J Alloys Compd. 2011;509:9019–25.
Zhang LC, Xu J, Eckert J. Thermal stability and crystallization kinetics of mechanically alloyed TiC/Ti-based metallic glass matrix composite. J Appl Phys. 2006;100:033514.
Cao QP, Li JW, Li JF, Zhou YH, Wang XD, Jiang JZ. Isochronal crystallization kinetics of Cu60Zr20Ti20 bulk metallic glass. J NonCryst Solids. 2011;357:1182–7.
Prajapati SR, Sauthor K, Ashmi TP, Pratap A. Non-isothermal crystallization kinetics of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim. 2016;124:21–33.
Ranganathan S, Heimendahl MV. The three activation energies with isothermal transformations: applications to metallic glasses. J Mater Sci. 1981;16:2401–4.
Wang WH, Dong C, Shek CH. Bulk metallic glasses. Mater Sci Eng R Rep. 2004;44(2):45–89.
Jin JS, Li FW, Yin G, Wang XY, Gong P. Influence of substitution of Cu by Ni on the crystallization kinetics of TiZrHfBeCu high entropy bulk metallic glass. Thermochim Acta. 2020;690:178650.
Acknowledgements
This work was financially supported by the General Research Project of Shaanxi Provincial Education Department (No. 22JK0418).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, K., Li, B., Fan, Xh. et al. Investigation of crystallization behavior of Hf26Be18Ti18Zr18Cu7.5Ni12.5 high mixing entropy amorphous alloys. J Therm Anal Calorim 148, 689–696 (2023). https://doi.org/10.1007/s10973-022-11778-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11778-7