Abstract
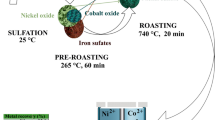
African cobalt-rich copper sulfide ore (CRCS) is an important copper-cobalt resource. The CRCS mainly contains chalcopyrite (CuFeS2), pyrite (FeS2), and carrollite (CuCo2S4). In our previous study, activated roasting was used to treat CRCS, converting CuCo2S4 into sulfate that is easy to leach Cu and Co. It was found that the kinetics of CuCo2S4 in CRCS transformation was slow during roasting, which is a key factor affecting the subsequent increase in cobalt leaching rate. Therefore, it is of practical significance to explore the kinetics process in the activated roasting process of CRCS. In this study, thermal roasting kinetics of CRCS in the air atmosphere was studied by TG-DTG method at heating rates of 5, 10, 15, and 20 K min−1, respectively. Meanwhile, the phase transformation behavior of major minerals, pyrite, and carrollite during roasting was studied. The results show that the roasting process of CRCS mainly goes through three stages: Stage I: removal of free water and crystal water; Stage II: oxidation of sulfide minerals to sulfate; and Stage III: decomposition of sulfate to oxide. Given Stage II, the deconvolution separation method was used to separate overlapping reaction peaks of FeS2 and CuCo2S4. The kinetic parameters were evaluated by Friedman method, KAS method, FWO method, and CR method, respectively. The most probable mechanism function and activation energy (E) were determined by comparing the model-free method with the model-fitting method. Results showed that the oxidation of FeS2 in CRCS conforms to the 2-D diffusion model; the oxidation of CuCo2S4 in CRCS accords with the Avrami–Eroféev model. Finally, thermodynamic parameters of the reaction including enthalpy, entropy, and Gibbs free energy were calculated. Kinetic analysis shows that the heating rate has a significant influence on the ore phase transition during the oxidation process of CRCS. The oxidation processes of different ore in CRCS conform to different kinetic mechanisms.







Similar content being viewed by others
Abbreviations
- CRCS:
-
Cobalt-rich copper sulfide ore
- FWO:
-
Flynn–Wall–Ozawa method
- KAS:
-
Kissinger–Akahira–Sunose method
- CR:
-
Coats–Redfern method
- E :
-
Activation energy (kJ mol−1)
- A :
-
Exponential factor (min−1)
- α :
-
Reaction conversion fraction
- β :
-
Heating rate (K min−1)
- f(α):
-
Differential forms of kinetic mechanism function
- G(α):
-
The integral form of kinetic mechanism function
- TAK:
-
Thermal roasting kinetics
- EV:
-
Electric vehicles
References
Shekhar AR, Parekh MH, Pol VG. Worldwide ubiquitous utilization of lithium-ion batteries: What we have done, are doing, and could do safely once they are dead? J Power Sources. 2022;523:231015. https://doi.org/10.1016/j.jpowsour.2022.231015.
Liu Y, Yu H, Wang Y, Tang D, Qiu W, Li W. Microwave hydrothermal renovating and reassembling spent lithium cobalt oxide for lithium-ion battery. Waste Manag. 2022;143:186–94. https://doi.org/10.1016/j.wasman.2022.02.024.
Dehaine Q, Tijsseling LT, Glass HJ, Törmänen T, Butcher AR. Geometallurgy of cobalt ores: a review. Miner Eng. 2021;160:106656. https://doi.org/10.1016/j.mineng.2020.106656.
Chan KH, Malik M, Azimi G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour Conserv Recycl. 2022;178:106076. https://doi.org/10.1016/j.resconrec.2021.106076.
Lme. London metal exchange: Cobalt.; 2022. [https://www.lme.com/]; URL https://www.lme.com/en-GB/Metals/Minor-metals/Cobalt#tabIndex=2 (accessed 2022.04.09)
Crundwell FK, du Preez NB, Knights BDH. Production of cobalt from copper-cobalt ores on the African Copperbelt -an overview. Miner Eng. 2020;156: 106450. https://doi.org/10.1016/j.mineng.2020.106450.
Cailteux JLH, Kampunzu AB, Lerouge C, Kaputo AK, Milesi JP. Genesis of sediment-hosted stratiform copper–cobalt deposits, central African Copperbelt. J Afr Earth Sci. 2005;42(1–5):134–58. https://doi.org/10.1016/j.jafrearsci.2005.08.001.
Davenport FKCS. Production of cobalt from the copper–cobalt ores of the central african copperbelt. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; 2011; 1: 377–91.
Tijsseling LT, Dehaine Q, Rollinson GK, Glass HJ. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Miner Eng. 2019;138:246–56. https://doi.org/10.1016/j.mineng.2019.04.022.
Dehaine Q, Filippov LO, Filippova IV, Tijsseling LT, Glass HJ. Novel approach for processing complex carbonate-rich copper-cobalt mixed ores via reverse flotation. Miner Eng. 2021. https://doi.org/10.1016/j.mineng.2020.106710.
Cui F, Mu W, Wang S, Xin H, Xu Q, Zhai Y, et al. Sodium sulfate activation mechanism on co-sulfating roasting to nickel-copper sulfide concentrate in metal extractions, microtopography and kinetics. Miner Eng. 2018;123:104–16. https://doi.org/10.1016/j.mineng.2018.04.013.
Guangshi Li HCXZ. Phase transformation and element migration in the oxidation process of nickel-copper sulfide ore. In: TMS 2015 144th anuual meeting & exhibition. Orlando, FL, USA. 2015.
Dunn JAKC. A TG/MS and DTA study of the oxidation of pentlandite. J Therm Anal Calorim. 1980;1(18):147–54.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Xia W, Wang S, Wang H, Xu T. Thermal effects of asphalt SARA fractions, kinetic parameter calculation using isoconversional method and distribution models. J Thermal Anal Calorim. 2021;146(4):1577–92. https://doi.org/10.1007/s10973-020-10152-9.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23. https://doi.org/10.1016/j.tca.2014.05.036.
Janković B, Adnađević B, Jovanović J. Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: Thermogravimetric analysis. Thermochim Acta. 2007;452(2):106–15. https://doi.org/10.1016/j.tca.2006.07.022.
Budrugeac P. An iterative model-free method to determine the activation energy of heterogeneous processes under arbitrary temperature programs. Thermochim Acta. 2011;523(1–2):84–9. https://doi.org/10.1016/j.tca.2011.05.003.
Blanco D, Oulego P, Ramos D, Fernández B, Cuetos JM. Model-free kinetics applied to evaluate the long-term thermal stability of three [NTf2] anion-based ionic liquids. Thermochim Acta. 2017;656:70–84. https://doi.org/10.1016/j.tca.2017.08.002.
Blaine RL, Kissinger HE. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6. https://doi.org/10.1016/j.tca.2012.04.008.
Li H, Liu S, Zhao J, Li D, Yuan Y. Thermal degradation behaviors of polydimethylsiloxane-graft-poly(methyl methacrylate). Thermochim Acta. 2013;573:32–8. https://doi.org/10.1016/j.tca.2013.09.014.
Berčič G. The universality of Friedman’s isoconversional analysis results in a model-less prediction of thermodegradation profiles. Thermochim Acta. 2017;650:1–7. https://doi.org/10.1016/j.tca.2017.01.011.
Wang J, Laborie MG, Wolcott MP. Comparison of model-free kinetic methods for modeling the cure kinetics of commercial pheno—formaldehyde resins. Thermochim Acta. 2005;439(1–2):68–73. https://doi.org/10.1016/j.tca.2005.09.001.
Jain AA, Mehra A, Ranade VV. Processing of TGA data: analysis of isoconversional and model fitting methods. Fuel. 2016;165:490–8. https://doi.org/10.1016/j.fuel.2015.10.042.
Mian I, Li X, Jian Y, Dacres OD, Zhong M, Liu J. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison. Bioresour Technol. 2019;294:122099. https://doi.org/10.1016/j.biortech.2019.122099.
Naqvi SR, Tariq R, Hameed Z, Ali I, Naqvi M, Chen W. Pyrolysis of high ash sewage sludge: kinetics and thermodynamic analysis using Coats-Redfern method. Renew Energy. 2019;131:854–60. https://doi.org/10.1016/j.renene.2018.07.094.
Huang M, Lv S, Zhou C. Thermal decomposition kinetics of glycine in nitrogen atmosphere. Thermochim Acta. 2013;552:60–4. https://doi.org/10.1016/j.tca.2012.11.006.
Ruan S, Xing P, Wang C, Chen Y, Yin F, Jie X. Thermal decomposition kinetics of arsenopyrite in arsenic-bearing refractory gold sulfide concentrates in nitrogen atmosphere. Thermochim Acta. 2020;690:178666. https://doi.org/10.1016/j.tca.2020.178666.
Ruan S, Wang C, Jie X, Yin F, Zhang Y, Yao Z. Kinetics of pyrite multi-step thermal decomposition in refractory gold sulphide concentrates. J Thermal Anal Calorim. 2022;147(5):3689–702. https://doi.org/10.1007/s10973-021-10761-y.
Müsellim E, Tahir MH, Ahmad MS, Ceylan S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl Thermal Eng. 2018;137:54–61. https://doi.org/10.1016/j.applthermaleng.2018.03.050.
Becker M, Villiers JD, Bradshaw D. The flotation of magnetic and non-magnetic pyrrhotite from selected nickel ore deposits. Miner Eng. 2010;23(11–13):1045–52. https://doi.org/10.1016/j.mineng.2010.07.002.
Shah SEKI. Oxidative roasting of covellite with minimal retardation from the CuO⋅CuSO4 film. Metall Trans. 1970;1:251–2156. https://doi.org/10.1007/bf02643428.
Gadalla AM. Kinetics of thermal decomposition of CuSO4 · 5H2O to CuO. Int J Chem Kinet. 1984;6(16):655–68. https://doi.org/10.1002/kin.550160604.
Ingraham TR, Marier P. The kinetics of the thermal decomposition of CoSO4 and Co3O4. Thermochim Acta. 1970;1(1):39–49. https://doi.org/10.1016/0040-6031(70)85027-4.
Xiao R, Yang W, Cong X, Dong K, Xu J, Wang D, et al. Thermogravimetric analysis and reaction kinetics of lignocellulosic biomass pyrolysis. Energy. 2020;201:117537. https://doi.org/10.1016/j.energy.2020.117537.
Manić N, Janković B, Pijović M, Waisi H, Dodevski V, Stojiljković D. Apricot kernel shells pyrolysis controlled by non-isothermal simultaneous thermal analysis (STA). J Thermal Anal Calorim. 2020;142(2):565–79. https://doi.org/10.1007/s10973-020-09307-5.
Yang F, Liao L, Zhao C, Tian Y. Combined kinetic analysis of overlapping multistep thermal decomposition of 5-nitro-2,4,6-triaminopyrimidine -1,3-di-N-oxide (ICM-102). Thermochim Acta. 2020;690:178663. https://doi.org/10.1016/j.tca.2020.178663.
Alves JLF, Da Silva JCG, Da Silva Filho VF, Alves RF, de Araujo Galdino WV, Andersen SLF. Determination of the bioenergy potential of brazilian Pine-Fruit shell via pyrolysis kinetics, thermodynamic study, and evolved gas analysis. Bioenerg Res. 2019;12(1):168–83. https://doi.org/10.1007/s12155-019-9964-1.
Hidayat S, Bakar MSA, Ahmed A, Iryani DA, Hussain M, Jamil F. Comprehensive kinetic study of Imperata Cylindrica pyrolysis via Asym2sig deconvolution and combined kinetics. J Anal Appl Pyrolysis. 2021;156:105133. https://doi.org/10.1016/j.jaap.2021.105133.
Mumbach GD, Alves JLF, Da Silva JCG, De Sena RF, Marangoni C, Machado RAF. Thermal investigation of plastic solid waste pyrolysis via the deconvolution technique using the asymmetric double sigmoidal function: determination of the kinetic triplet, thermodynamic parameters, thermal lifetime and pyrolytic oil composition for clean energy recovery. Energ Convers Manage. 2019;200: 112031. https://doi.org/10.1016/j.enconman.2019.112031.
Jamieson HE, Walker SR, Parsons MB. Mineralogical characterization of mine waste. Appl Geochem. 2015;57:85–105. https://doi.org/10.1016/j.apgeochem.2014.12.014.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFC1908401), the National Natural Science Foundation of China (52034002, U1802253), and the Fundamental Research Funds for the Central Universities (FRF-TT-19-001).
Author information
Authors and Affiliations
Contributions
WY contributed to investigation, writing—review and editing, formal analysis, resources. XL contributed to investigation, conceptualization, writing—original draft. YL contributed to validation, visualization. BM contributed to conceptualization, writing—review and editing, project administration. HW contributed to formal analysis, resources, writing—review and editing. XJ contributed to conceptualization, visualization. Chengyan Wang contributed to project administration, funding acquisition and supervision.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, W., Li, X., Liu, Y. et al. Thermal roasting behavior and kinetics of African cobalt-rich copper sulfide ore in air atmosphere. J Therm Anal Calorim 147, 13469–13481 (2022). https://doi.org/10.1007/s10973-022-11628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11628-6