Abstract
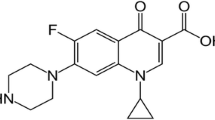
Chitosan (CTS) and naproxen (NAP) salts were prepared using different reaction parameters such as time, molar ratio and temperature. The salt was also synthesized from chitosan crosslinked with epichlorohydrin (EP). The starting reagents and salts were characterized by spectroscopic (1H NMR and FTIR) and thermoanalytical (TG/DTG/DTA, DSC and TG-FTIR) techniques. Under dry air atmosphere, the CTS TG/DTG curve showed three mass loss events, one resulting from dehydration and the other two referring to the decomposition and burning of the carbonized material, with a residue of 5.95% at 1000 °C. Under the same conditions, the DTA curve exhibited an endothermic dehydration peak and an exothermic decomposition peak. TG/DTG curve of NAP shows two stages of mass loss, the first related to the drug decomposition and the other of burning the carbonized material. The DTA curve showed an endothermic peak at 156.7 °C (melting), in addition to other endo and exothermic peaks attributed to evaporation, decomposition and burning of the carbonized material. The TG/DTG/DTA curves of naproxen salts with non-crosslinked (CN) and crosslinked (CEPN) chitosan showed profiles similar to those of CTS; however, the mass loss events were observed at different temperature intervals, suggesting an interaction between CTS and NAP. The DSC curves of CNs and CEPNs showed salt formation, as the endothermic peak characteristic of NAP melt was not observed. These data are supported by spectroscopic techniques (FTIR and 1H NMR). In experiments involving TG-FTIR, the release of gases was observed: CO, CO2, CH3CONH2, CH3COOH, NH3 and CH4.








Similar content being viewed by others
References
Pandey A. Pharmaceutical and biomedical applications of cellulose nanofbers: a review. Environ Chem Lett. 2021;19:2043–55.
Wagh M, Waghmare S, Kamble H. A review: drug- excipient interactions study. World J Pharm Res. 2021;10(12):214–24.
Si Y, Luo H, Zhou F, Bai X, Han L, Sun H, Cha R. Advances in polysaccharide nanocrystals as pharmaceutical excipientes. Carbohydr Polym. 2021;262:117922.
Pooresmaeil M, Namazi H. Developments on carboxymethyl starch-based smart systems as promising drug carriers: A review. Carbohydr Polym. 2021;258:117654.
Uwah TO, Akpabio EI, Effiong DE. Plants as pharmaceutical excipients in oral sustained drug delivery systems: a review. J Appl Pharm Res. 2021;9(3):26–38.
Recife ACD, Meneguin AB, Cury BSF, Evangelista RC. Evaluation of retrograded starch as excipient for controlled release matrix tablets. J Drug Del Sci Technol. 2017;40:83–94.
Beneke CE, Viljoen AM, Hamman JH. Polymeric plant-derived excipients in drug delivery. Molecules. 2009;14:2602–20.
Basha SK, Muzammil MS, Dhandayuthabani R, Kumari SV. Polysaccharides as excipient in drug delivery system. Mater Today: Proc. 2021;36:280–9.
Vishakha SK, Kishor DB, Sudha SR. Natural polymers–a comprehensive review. Int J Res Pharm Biomed Sci. 2012;3(4):1597–613.
Hartesi B, Sriwidodo Marline A, Chaerunisaa AY. Starch as Pharmaceutical Excipient. Int J Pharm Sci Rev Res. 2016;41(2):59–64.
Villanova JCO, Oréfice RL, Cunha AS. Aplicações farmacêuticas de polímeros. Polímeros. 2010;20(1):51–64.
dos Santos JE, Dockal ER, Cavalheiro ETG. Synthesis and characterization of Schiff bases from chitosan and salicylaldehyde derivatives. Carbohyd Polym. 2005;60:277–82.
Antony R, David ST, Karuppasamy K, Sanjeev G, Balakumar S. Synthesis, spectroscopic and catalytic studies of Cu (II), Co (II) and Ni (II) complexes immobilized on Schiff base modified chitosan. J Mol Struct. 2013;1050:53–60.
Majeti NV, Kumar R. A review of chitin and chitosan applications. React Funct Polym. 2000;26:1–27.
Roy BC. Potential aspects of chitosan as pharmaceutical excipient. Acta Pol Pharm. 2011;68(5):619–22.
Agnirotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro and nanoparticles in drug delivery. J Control Release. 2004;100:5–28.
Prabaharan M. Chitosan-based nanopaticles for tumor-targed drug delivery. Int J Biol Macromol. 2015;72:1313–22.
Peter MG. Applications and environmental aspects of chitin and chitosan. J Macromol Sci A. 1995;32:629–40.
Qandil AM, Obaidat AA, Ali MAM, Al-Taani BM, Tashtoush BM, Al-Jbour ND, Al-Remawi MM, Al-Sou’od KA, Badwan AA. Investigation of the interactions in complexes of low molecular weight chitosan with ibuprofen. J Solut Chem. 2009;38:695–712.
Imai T, Shiraishi S, Saitô H, Otagiri M. Interaction of indomethacin with low molecular weight chitosan and improvements of some pharmaceutical properties of indomethacin by low molecular weight chitosan. Int J Pharm. 1991;67:11–20.
Zerrouk N, Mennini N, Maestrelli F, Chemtob C, Mura P. Comparison of the effect of chitosan and polyvinylpyrrolidone on dissolution properties and analgesic effect of naproxen. Eur J Pharm Biopharm. 2004;57:93–9.
Perchyonok VT, Reher V, Zhang S, Grobler SR, Oberholzer TG, Massey W. Insights and relative effect of aspirin, naproxen and ibuprofen containing hydrogels: from design to performance as a functional dual capacity restorative material and build in free radical defense: In-vitro studies. Open J Stomatol. 2014;4:73–83.
Meller J. The influence of different kinds of chitosan on bioavailability of anti-inflammatory drugs. Prog Chem Appl Chitin Deriv. 2010;15:127–34.
Gouda M, Elayaan U, Youssef MM. Synthesis and biological activity of drug delivery system based on chitosan nanocapsules. Adv Nanoparticles. 2014;3:148–58.
Mohammed T, Ways M, Lau WM, Khutoryanskiy IVV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018;10:267.
Boonsongrit Y, Mueller BW, Mitrevej A. Characterization of drug–chitosan interaction by 1H NMR, FTIR and isothermal titration calorimetry. Eur J Pharm Biopharm. 2008;69:388–95.
El Mouelhi M, Ruelius HW, Fenselau C, Dulik DM. Species-dependent enantioselective glucuronidation of three 2-arylpropionic acids. naproxen, ibuprofen, and benoxaprofen. Drug Metab Dispos. 1987;15:767–72.
Signini R, Campana Filho SP. Purificação e caraterização de quitosana comercial. Polímeros. 1998;8:63–8.
Signini R, Campana Filho SP. Característica e propriedades de quitosanas purificadas nas formas neutra, acetato e cloridrato. Polímeros. 2001;11:58–64.
Silva RC, Andrade Junior MA, Cestari AR. Adsorção de Cr(VI) em esferas reticuladas de quitosana: novas correlações cinéticas e termodinâmicas utilizando microcalorimetria isotérmica contínua. Quim Nova. 2010;33:880–4.
Hirai A, Odani H, Nakajima A. Determination of degree of deacetilation of chitosan by 1H NMR spectroscopy. Polym Bull. 1991;26:87–94.
Guinesi LS, Cavalheiro ETG. Influence of some reactional parameters on the substitution degree of biopolymeric schiff bases prepared from chitosan and salicylaldehyde. Carbohydr Polym. 2006;65:557–61.
Carignani E, Borsacchi S, Bradley JP, Brown SP, Geppi M. Strong intermolecular ring current influence on 1H chemical shifts in two crystalline forms of naproxen: a combined solid-state NMR and DFT study. J Phys Chem C. 2013;117:17731–40.
Yakub Iqbal MD, Narayana Rao KMV, Sridhar G, Padmanabha Raju P, Deshpande GR, Moses BJ. Characterization and relative response factor determination of process related impurity in Naproxen by nuclear magnetic resonance spectroscopy. J Pharm Biomed. 2011;56:484–90.
Duarte ML, Ferreira MC, Marvão MR, Rocha J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int J Biol Macromol. 2002;31:1–8.
Brugnerotto J, Lizardi J, Goycoolea FM, Arguelles-Monal W, Desbrières J, Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569–80.
Pereira FS, Lanfredi S, González ERP, da Silva Agostini DL, Gomes HM, dos Santos MR. Thermal and morphological study of chitosan metal complexes. J Therm Anal Calorim. 2017;129:291–301.
Silverstein RM, Webster FX, Kiemle DJ, Bryco DL. Spectrometric Identification of Organic Compounds. 8th ed. New York: Willey; 2005.
Araújo EL, Barbosa HFG, Dockal ER, Cavalheiro ETG. Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int J Biol Macromol. 2017;95:168–76.
Guilherme VA, Ribeiro LNM, Alcântara ACS, Castro SR, Rodrigues da Silva GH, da Silva CG, Breitkreitz MC, Clemente-Napimoga J, Macedo CG, Abdalla HB, Bonfante R, Cereda CMS, de Paula E. Improved efficacy of naproxen-loaded NLC for temporomandibular joint administration. Sci Rep. 2019;9:1–11.
Bannach G, Arcaro R, Ferroni DC, Siqueira AB, Treu-Filho O, Ionashiro M, Schnitzler E. Thermoanalytical study of some anti-inflammatory analgesic agents. J Therm Anal Calorim. 2010;102:163–70.
Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–31.
Pavia DL, Lampman GM, Kriz GS. Introduction to spectroscopy. 3rd ed. Boston: Thomson Learning; 2001.
Yoshida MI, Gomes ECL, Soares CDV, Cunha AF, Oliveira MA. Thermal analysis applied to verapamil hydrochloride characterization in pharmaceutical formulations. Molecules. 2010;15:2439–52.
United States. Department of Commerce. National Institute of Standards and Technology. Infrared spectroscopy standard reference data program collection. Gaithersburg: NIST, 2018.
Nicolet-ThermoScientific Co. Nicolet EPA Vapor Phase database. Ominic. 8.0 software. Madison: ThermoScientific.
Medeiros RS, Ferreira APG, Cavalheiro ETG. Thermal behavior of naproxen and ketoprofen non-steroidal anti-inflammatory drugs. J Therm Anal Calorim. 2020;142:849–59.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo,Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Contributions
RSM Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Roles/Writing-original draft; Writing-review & editing. APGF Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Roles/Writing-original draft; Writing-review & editing. ETGC Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration; Resources; Supervision; Validation; Visualization; Roles/Writing-original draft; Writing-review & editing.
Corresponding author
Ethics declarations
Conflict of interests
Authors declare that there are no directly or indirectly conflict of interested related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Medeiros, R.S., Ferreira, A.P.G. & Cavalheiro, E.T.G. Chitosan and naproxen salts: preparation and characterization. J Therm Anal Calorim 148, 177–190 (2023). https://doi.org/10.1007/s10973-022-11626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11626-8