Abstract
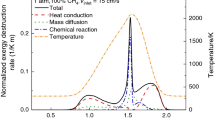
A novel method for estimating the non-equilibrium entropy generated in a premixed stretched methane flame is proposed. Counter-flow premixed methane flames were numerically investigated. The flame structure in terms of species, species production rate, and temperature was modeled using the San Diego mechanism with multicomponent diffusion. The local entropy generation of flames due to heat conduction, mass diffusion, viscous dissipation, and chemical reaction and the total irreversibility induced were analyzed for flames with various equivalence ratios at various temperatures, pressures, and counter-flow strain rates. The strain rate had a weak effect on the mass diffusion irreversibility and thermal conduction irreversibility but a strong effect on the chemical irreversibility. The overall irreversibility was highest at the equivalence ratio of 1.1. The heat conduction irreversibility, mass diffusion irreversibility, and chemical irreversibility all increased as the pressure was increased, and the chemical irreversibility increased as the temperature was increased. In all studied cases, the flame thickness decreased as the pressure or temperature was increased, and the flame thickness was inversely related to the non-equilibrium irreversibility. An empirical formula for predicting the irreversibility as a function of flame thickness was derived. The formula is valid not only for pure methane premixed flames but also for binary or triply blended fuel mixtures of methane, carbon monoxide, and hydrogen.















Similar content being viewed by others
Abbreviations
- a :
-
Strain rate (s−1)
- D :
-
Diffusivity (m2s−1)
- F :
-
Function of x denotes axial velocity
- G :
-
Function of x denotes radial velocity
- H :
-
Pressure derivative in radial
- h :
-
Specific enthalpy (kJ kg−1)
- i :
-
Specific irreversibility (kJ kg−1)
- I :
-
Total specific irreversibility (kJ kg−1)
- P :
-
pressure (atm)
- R :
-
Universal gas constant (kJ K−1 kmol−1)
- r :
-
Radial direction
- s :
-
Specific entropy (kJ kg−1 K−1)
- T :
-
Temperature (K)
- u :
-
Axial velocity of counter-flow premixed flame (m s−1)
- v :
-
Radial velocity of counter-flow premixed flame s−1
- W :
-
Molar weight (g mol−1)
- X :
-
Molar fraction
- x :
-
Axial direction
- Y :
-
Mass fraction
- δ :
-
Flame thickness (mm)
- λ :
-
Thermal conductivity (W m−1 K−1)
- μ :
-
Chemical potential (kJ kg−1)
- ρ :
-
Density (kg m−3)
- τ :
-
Viscous stress tensor
- ϕ :
-
Equivalence ratio
- ω :
-
Reaction rate (kmol m−3 s−1)
- 0:
-
Properties at standard state
- chem:
-
Chemistry
- cond:
-
Conduction
- diff:
-
Mass diffusion
- flame:
-
Flame
- in:
-
Reactants at inlet
- k:
-
Species k
- max:
-
Maximum
- mix:
-
Mixtures
- visc:
-
Viscosity
- x:
-
Axial direction of the counter-flow burner
- ‧ :
-
Dot over symbol denotes time rate
- _ :
-
Bar over symbol denotes average
- T :
-
Thermal
References
Parsa S, Neshat E. Thermodynamic and statistical analysis on the effect of exhaust gas recirculation on waste heat recovery from homogeneous charge compression ignition engines. J Therm Anal Calorim. 2022;147:6349–61. https://doi.org/10.1007/s10973-021-10923-y.
Lu JJ, Chen WH. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl Eng. 2015;160:49–57. https://doi.org/10.1016/j.apenergy.2015.09.026.
Naqvi SR, Tariq R, Hameed Z, Ali I, Naqvi M, Chen WH, Ceylan S. Pyrolysis of high ash sewage sludge: kinetics and thermodynamic analysis using Coats-Redfern method. Renew Energ. 2019;131:854–60. https://doi.org/10.1016/j.renene.2018.07.094.
Chen WH, Chen KH, Ubando AT, Lee WJ, Chio MH. Redox degrees of iron-based oxygen carriers in cyclic chemical looping combustion using thermodynamic analysis. Chem Eng J. 2021;426:130834. https://doi.org/10.1016/j.renene.2018.07.094.
Lakhfif F, Nemouchi Z, Mebarek-Oudina F. Numerical investigation of the different spray combustion models under diesel condition. Int J Appl Eng Res. 2016;11(18):9393–9.
Sciacovelli A, Verda V, Sciuba E. Entropy generation analysis as a design tool- a review. Renew Sustain Energy Rev. 2015;43:1167–81. https://doi.org/10.1016/j.rser.2014.11.104.
Dincer I, Rosen MA. Exergy: energy, environment and sustainable development. Oxford: Elsevier Science; 2013.
Wu CY, Yu WC, Cheng CC. Characteristics of dimethyl ether oxidation in a preheated Pt-γ-Al2O3 catalytic reactor. Combust Sci Technol. 2020. https://doi.org/10.1080/00102202.2020.1748608.
Karki P, Perumal DA, Yadav AK. Comparative studies on air, water and nanofluids based Rayleigh-Benard natural convection using lattice Boltzmann method: CFD and exergy analysis. J Therm Anal Calorim. 2022;147:1487–503. https://doi.org/10.1007/s10973-020-10496-2.
Shinde BJ, Karunamurthy K, Ismail S. Thermodynamic analysis of gasoline-fueled electronic fuel injection digital three-spark ignition (EFI-DTSI) engine. J Therm Anal Calorim. 2022;141:2355–67. https://doi.org/10.1007/s10973-020-10120-3.
Koruyucu E, Ekici S, Karakoc TH. Performing thermodynamic analysis by simulating the general characteristics of the two-spool turbojet engine suitable for drone and UAV propulsion. J Therm Anal Calorim. 2021;145:1303–15. https://doi.org/10.1007/s10973-020-10449-9.
Wu CY, Hu BY. Charaterisation of the effect of water content on the methanol spray combustion. Int J Exergy. 2022;38(2):139–57. https://doi.org/10.1504/IJEX.2022.123596.
Hajibashi FA, Arabkoohsar A, Babaelahi M. Risk assessment, dynamic analysis and multi-objective optimization of a solar-driven hybrid gas/steam power plant. J Therm Anal Calorim. 2021;145:955–71. https://doi.org/10.1007/s10973-020-10221-z.
Nourpour M, Manesh MHK. Modeling and 6E analysis of a novel quadruple combined cycle with turbocompressor gas station. J Therm Anal Calorim. 2021;147:5165–97. https://doi.org/10.1007/s10973-021-10898-w.
Wu CY, Chen KH, Yang SY. Experimental study of porous metal burners for domestic stove applications. Energy Convers Manag. 2014;77:380–8. https://doi.org/10.1016/j.enconman.2013.10.002.
İlbaş M, Karyeyen S. Modelling of combustion performances and emission characteristics of coal gases in a model gas turbine combustor. Int J Energy Res. 2014;38:1171–80. https://doi.org/10.1002/er.3135.
Cheng TS, Chao YC, Chen CP, Wu CY. Further analysis of chemical kinetic structure of a standoff microjet methane diffusion flame near extinction. Combust Flame. 2008;152(3):461–7. https://doi.org/10.1016/j.combustflame.2007.10.007.
Aissa A, Slimani MEA, Mebarek-Oudina F, Fares R, Zaim A, Kolsi L, Sahnoun M, Ganaoui ME. Pressure-driven gas flows in micro channels with a slip boundary: A numerical investigation. Fluid Dyn Mater Process. 2020;16(2):147–59. https://doi.org/10.32604/fdmp.2020.04073.
Arpaci VS, Selamet A. Entropy production in flames. Combust Flame. 1988;73(3):251–9. https://doi.org/10.1016/0010-2180(88)90022-3.
Dunbar WR, Lior N. Sources of combustion irreversibility. Combust Sci Technol. 1994;103:41–63. https://doi.org/10.1080/00102209408907687.
Nishida K, Takagi T, Kinoshita S. Analysis of entropy generation and exergy loss during combustion. Proc Combust Inst. 2002;29:869–74. https://doi.org/10.1016/S1540-7489(02)80111-0.
Liu Y, Zhang J, Ju D, Huang Z, Han D. Analysis of exergy losses in laminar premixed flames of methane/hydrogen blends. Int J Hydrog Energy. 2019;44:24043–53. https://doi.org/10.1016/j.ijhydene.2019.07.123.
Zhang J, Han D, Huang Z. Second-law thermodynamic analysis for premixed hydrogen flames with diluents of argon/nitrogen/carbon dioxide. Int J Hydrog Energy. 2019;44:5020–9. https://doi.org/10.1016/j.ijhydene.2019.01.041.
Acampra L, Marra FS. Second law thermodynamic analysis of syngas premixed flames. Int J Hydrog Energy. 2020;45:12185–202. https://doi.org/10.1016/j.ijhydene.2020.02.142.
Zhang J, et al. Second-law thermodynamic analysis in premixed flames of ammonia and hydrogen binary fuels. J Eng Gas Turbines Power. 2019;141:071007. https://doi.org/10.1115/1.4042412.
Liu Y, et al. Second-law thermodynamic analysis on non-premixed counterflow methane flames with hydrogen addition. J Therm Anal Calorim. 2020;139:2577–83. https://doi.org/10.1007/s10973-019-08583-0.
Kim N, Kim Y, Jaafar MNM, Rahim MR, Said M. Effects of hydrogen addition on structure and NO formation of highly CO-Rich syngas counterflow nonpremixed flames under MILD combustion regime. Int J Hydrog Energy. 2021;46:10518–34. https://doi.org/10.1016/j.ijhydene.2020.12.120.
Liu Y, Chen S, Yang B, Liu K, Zheng C. First and second thermodynamic-law comparison of biogas MILD oxy-fuel combustion moderated by CO2 or H2O. Energy Convers Manag. 2015;106:625–34. https://doi.org/10.1016/j.enconman.2015.09.076.
Liu D, Wang H, Zhang Y, Liu H, Zheng Z, Yao M. On the entropy generation and exergy loss of laminar premixed flame under engine-relevant conditions. Fuel. 2021;283:119245. https://doi.org/10.1016/j.fuel.2020.119245.
Jiang D, Yang W, Chua KJ. Entropy generation analysis of H2/air premixed flame Ćin micro-combustors with heat recuperation. Chem Eng Sci. 2013;98:265–72. https://doi.org/10.1016/j.ces.2013.05.038.
Jiang D, Yang W, Teng J. Entropy generation analysis of fuel lean premixed CO/H2/air flames. Int J Hydrog Energy. 2015;40:5210–20. https://doi.org/10.1016/j.ijhydene.2015.02.082.
Wang W, Zuo Z, Liu J, Yang W. Entropy generation analysis of fuel premixed CH4/H2/air flames using multistep kinetics. Int J Hydrog Energy. 2016;41:20744–52. https://doi.org/10.1016/j.ijhydene.2016.08.103.
Zuo W, Zhang Y, Li J, Li Q, He Z. A modified micro reactor fueled with hydrogen for reducing entropy generation. Int J Hydrog Energy. 2019;44:27984–94. https://doi.org/10.1016/j.ijhydene.2019.09.009.
Datta A. Effects of gravity on structure and entropy generation of confined laminar diffusion flames. Int J Therm Sci. 2005;44:429–40. https://doi.org/10.1016/j.ijthermalsci.2004.10.003.
Briones AM, Mukhopadhyay A, Aggarwal SK. Analysis of entropy generation in hydrogen-enriched methane–air propagating triple flames. Int J Hydrog Energy. 2009;34:1074–83. https://doi.org/10.1016/j.ijhydene.2008.09.103.
Dressler L, Nicolai H, Agrebi S, Ries F, Sadiki A. Computation of entropy production in stratified flames based on chemistry tabulation and an eulerian transported probability density function approach. Entropy. 2022;24:615. https://doi.org/10.3390/e24050615.
Yan H, Tang G, Wang C, Li L, Zhou Y, Zhang Z. Thermodynamics irreversibilities analysis of oxy-fuel diffusion flames: the effect of oxygen concentration. Entropy. 2022;24:205. https://doi.org/10.3390/e24020205.
Ghai SK, Ahmed U, Chakraborty N, Klein M. Entropy generation during head-on interaction of premixed flames with inert walls within turbulent boundary layers. Entropy. 2022;24:463. https://doi.org/10.3390/e24040463.
Rakopoulos CD, Michos CN. Generation of combustion irreversibilities in a spark ignition engine under biogas–hydrogen mixtures fueling. Int J Hydrog Energy. 2009;34:4422–37. https://doi.org/10.1016/j.ijhydene.2009.02.087.
Chen S. Analysis of entropy generation in counter-flow premixed hydrogen–air combustion. Int J Hydrog Energy. 2010;35:1401–11. https://doi.org/10.1016/j.ijhydene.2009.11.080.
Chen S, Li J, Han H, Liu Z, Zheng C. Effects of hydrogen addition on entropy generation in ultra-lean counter-flow methane-air premixed combustion. Int J Hydrog Energy. 2010;35:3891–902. https://doi.org/10.1016/j.ijhydene.2010.01.120.
Chen S, Han H, Liu Z, Li J, Zheng C. Analysis of entropy generation in non-premixed hydrogen versus heated air counter-flow combustion. Hydrog Energy. 2010;35:4736–46. https://doi.org/10.1016/j.ijhydene.2010.02.113.
Chen S, Mi J, Liu H, Zheng C. First and second thermodynamic-law analyses of hydrogen-air counter-flow diffusion combustion in various combustion modes. Hydrog Energy. 2012;37:5234–45. https://doi.org/10.1016/j.ijhydene.2011.12.039.
Safer K, Ouadha A, Tabet F. Entropy generation in turbulent syngas counterflow diffusion flames. Int J Hydrog Energy. 2017;42:29532–44. https://doi.org/10.1016/j.ijhydene.2017.08.217.
Johnson RF, VanDine AC, Esposito GL, Chelliah HK. On the axisymmetric counterflow flame simulations: is there an optimal nozzle diamter and separation distance to apply quasi one dimentsional theory? Combust Sci Technol. 2015;187:37–59. https://doi.org/10.1080/00102202.2014.972503.
Lockett RD, Boulanger B, Harding SC, Greenhalgh DA. The structrue and stability of the lmainar counter-flow partially premixed methane/air triple flame. Combust Flame. 1999;119(1–2):109–20. https://doi.org/10.1016/S0010-2180(99)00046-2.
Puri IK, Seshadri K. The extinction of counterflow premixed flames burning diluted methane-air, and diluted propane-air mixtures. Combust Sci Technol. 1987;53(1):55–65. https://doi.org/10.1080/00102208708947019.
Mokrin S, Fursenko R, Minaev S. Thermal-diffusive stability of counterflow premixed flames at low lewis numbers. Adv Mat Res. 2014;1040:608–13. https://doi.org/10.4028/www.scientific.net/AMR.1040.608.
San Diego Mecahnism. 2021. http://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html. Accessesed 16 Jul 2021.
Nakamura H, Takahashi H, Tezuka T, Hasegawa S, Maruta K, Abe K. Effects of CO-to-H2 ratio and diluents on ignition properties of syngas examined by weak flames in a micro flow reactor with a controlled temperature profile. Combust Flame. 2016;172:94–104. https://doi.org/10.1016/j.combustflame.2016.06.024.
Xie Y, Lv N, Li Q, Wang J. Effects of CO addition on laminar flame characteristics and chemical reactions of H2 and CH4 in oxy-fuel (O2/CO2) atmosphere. Int J Hydrog Energy. 2020;45:20472–81. https://doi.org/10.1016/j.ijhydene.2019.10.138.
Quattrocchi S, Aggarwal SK, Katta VR. Liftoff and blowout characteristics of laminar syngas nonpremixed flames. Int J Hydrog Energy. 2018;43:6421–33. https://doi.org/10.1016/j.ijhydene.2018.01.194.
Wu CK, Law CK. On the determination of laminar flame speeds from stretched flames. Symp Combust Proc. 1985;20(1):1941–9. https://doi.org/10.1016/S0082-0784(85)80693-7.
Lowry W, de Vries J, Krejci M, Petersen E, Serinyel Z, Metcalfe W, Curran H, Bourque G. Laminar flame speed measurements and modeling of pure alkanes and alkane blends at elevated pressures. J Eng Gas Turbines Power. 2011;133(9):091501. https://doi.org/10.1115/1.4002809.
Vagelopoulos CM, Egolfopoulos FN. Direct experimental determination of laminar flame speeds. Symp Combust Proc. 1998;27:513–9. https://doi.org/10.1016/S0082-0784(98)80441-4.
Gu XJ, Haq MZ, Lawes M, Woolley R. Laminar burning velocity and Markstein lengths of methane–air mixtures. Combust Flame. 2000;121:41–58. https://doi.org/10.1016/S0010-2180(99)00142-X.
Bosschaart KJ, de Goey LPH. The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method. Combust Flame. 2004;136:261–9. https://doi.org/10.1016/j.combustflame.2003.10.005.
Park O, Veloo PS, Liu N, Egolfopoulos N. Combustion characteristics of alternative gaseous fuels. Proc Combust Inst. 2011;33:887–94. https://doi.org/10.1016/j.proci.2010.06.116.
Rozenchan G, Zhu DL, Law CK, Tse SD. Outward propagation, burning velocities, and chemical effects of methane flames up to 60 ATM. Proc Combust Inst. 2002;29:1461–70. https://doi.org/10.1016/S1540-7489(02)80179-1.
Hassan MI, Aung KT, Faeth GM. Measured and predicted properties of laminar premixed methane/air flames at various pressures. Combust Flame. 1998;115:539–50. https://doi.org/10.1016/S0010-2180(98)00025-X.
Dirrenberger P, Gall HL, Bounaceur R, Herbinet O, Glaude P-A, Konnov A, Battin-Leclerc F. Measurements of laminar flame velocity for components of natural gas. Energy Fuels. 2011;25:3875–84. https://doi.org/10.1021/ef200707h.
Pizzuti L, Martins CA, dos Santos LR, Guerra DRS. Laminar burning velocity of methane/air mixtures and flame propagation speed close to the chamber wall. Energy Procedia. 2017;120:126–33. https://doi.org/10.1016/j.egypro.2017.07.145.
Wu CY, Chao YC, Cheng TS, Chen CP, Ho CT. Effects of CO addition on the characteristics of laminar premixed CH4/air opposed-jet flames. Combust Flame. 2009;156:362–73. https://doi.org/10.1016/j.combustflame.2008.10.028.
Cheng TS, Chang YC, Chao YC, Chen GB, Li YH, Wu CY. An experimental and numerical study on characteristics of laminar premixed H2/CO/CH4/air flames. Int J Hydrog Energy. 2011;36:13207–17. https://doi.org/10.1016/j.ijhydene.2011.07.077.
Acknowledgements
This research was supported by the Ministry of Science and Technology, Taiwan, under Grant no. MOST 110-2221-E-006 -093. We thank Research Center for Energy Technology and Strategy, National Cheng King University, for providing computational resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, CR., Wu, CY. An empirical formula to predict the overall irreversibility of counter-flow premixed flames of methane and its mixtures. J Therm Anal Calorim 147, 14587–14599 (2022). https://doi.org/10.1007/s10973-022-11573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11573-4