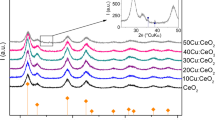
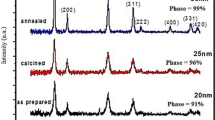
Abstract
In this work, we bring the first large-scale study of grain growth kinetics of copper-doped ceria nanoparticles. Since most of the notable applications of ceria nanoparticles, such as catalysis or solid oxide fuel cells, require its use at elevated temperatures, particle agglomeration and aggregation occur, which diminish the favorable properties of ceria. Doping with copper was chosen as a strategy for the increase of ceria thermal stability. Pure ceria and ceria doped with 10, 20, 30 and 40 mol.% of copper were prepared by hydrothermal synthesis, thermally treated at temperatures in the range between 300 and 700 °C at various annealing times and analyzed using X-ray diffraction analysis and transmission electron microscopy. An isothermal grain growth kinetics analysis was conducted based on the crystallite sizes calculated through the Scherrer equation in order to determine the influence of copper doping on ceria thermal stability. Three temperature ranges with different grain growth regimes and kinetic parameters were defined. The activation energies increase, while the grain growth exponents decrease from low to high temperature range, which is in concordance with the change in the growth mechanism from diffusion to Ostwald-Ripening. It was established that the thermal stability of ceria does not solely depend on the lattice defects introduced by copper, but also on microstructure, porosity and sample preparation. Still, the addition of copper has a positive influence on thermal stability of ceria up to 650 °C, and the sample with 40 mol.% of added copper has the slowest grain growth in the full studied temperature range.






Similar content being viewed by others
Availability of data and materials
All the data can be obtained directly from the authors.
Code availability
Not applicable.
References
Huang H, Xu Y, Feng Q, Leung DYC. Low temperature catalytic oxidation of volatile organic compounds: a review. Catal Sci Technol. 2015. https://doi.org/10.1039/C4CY01733A.
Kurajica S, Lučić Blagojević S. Introduction to nanotechnology. 1st ed. Zagreb: HDKI; 2017.
Groza JR. Nanosintering. Nanostruct Mater. 1999. https://doi.org/10.1016/S0965-9773(99)00284-6.
O’Hayre R. Materials kinetics fundamentals: principles, processes and applications. Hoboken: Wiley; 2015.
Mogensen M, Sammes NM, Tompsett GA. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000. https://doi.org/10.1016/S0167-2738(99)00318-5.
Melchionna M, Trovarelli A, Fornasiero P. Chapter 2: Synthesis and properties of cerium oxide-based materials. In: Scire S, Palmisano L, editors. Cerium oxide (CeO2): synthesis, properties and applications, in metal oxides. Amsterdam: Elsevier; 2020. p. 13–43.
Montini T, Melchionna M, Monai M, Fornasiero P. Fundamentals and catalytic applications of CeO2-based materials. Chem Rev. 2016. https://doi.org/10.1021/acs.chemrev.5b00603.
Sumi H, Shimada H, Yamaguchi Y, Yamaguchi T. Metal-supported microtubular solid oxide fuel cells with ceria-based electrolytes. J Ceram Soc Jpn. 2017. https://doi.org/10.2109/jcersj2.16221.
Pikalova EYU, Kolchugin AA, Bamburov VG. Ceria-based materials for high-temperature electrochemistry applications. Int J of Energy Prod Mgmt. 2016. https://doi.org/10.2495/EQ-V1-N3-272-283.
Singhal SC. Solid oxide fuel cells. Electrochem Soc Interface. 2007;16:41.
Ramachandran R, Chen TW, Chen SM, Raja P, Fernandez C, Rani SD, Gajendran P, Raju G, Baskar T, Jeyapragasam T. Highly enhanced electrochemical performance of novel based electrode materials for supercapacitor applications: an overview. Int J Electrochem Sci. 2019. https://doi.org/10.20964/2019.02.75.
Saravanan T, Shanmugam M, Anandan P, Azhagurajan M, Pazhanivel K, Arivanandhan M, Hayakawad Y, Jayavel R. Facile synthesis of graphene-CeO2 nanocomposites with enhanced electrochemical properties for supercapacitors. Dalton Trans. 2015. https://doi.org/10.1039/C5DT01235J.
Dezfuli AS, Ganjali MR, Naderi HR, Norouzi P. A high performance supercapacitor based on a ceria/graphene nanocomposite synthesized by a facile sonochemical method. RSC Adv. 2015. https://doi.org/10.1039/C5RA02957K.
Chen X, Paul R, Dai L. Carbon-based supercapacitors for efficient energy storage. Natl Sci Rev. 2017. https://doi.org/10.1093/nsr/nwx009.
Almar L, Tarancon A, Andreu T, Torrell M, Hu Y, Dezanneau G, Morata A. Mesoporous ceramic oxides as humidity sensors: a case study for gadolinium-doped ceria. Sens Actuators B Chem. 2015. https://doi.org/10.1016/j.snb.2015.04.018.
Divya T, Nikhila MP, Anju M, Arsha Kusumam TV, Akhila AK, Ravikiran YT, Renuka NK. Nanoceria based thin films as efficient humidity sensors. Sens Actuators A. 2017. https://doi.org/10.1016/j.sna.2017.05.008.
Jasinski P, Suzuki T, Anderson HU. Nanocrystalline undoped ceria oxygen sensor. Sens Actuators B Chem. 2003. https://doi.org/10.1016/S0925-4005(03)00407-6.
Ren Z, Peng F, Li J, Liang X, Chen B. Morphology-dependent properties of Cu/CeO2 catalysts for the water-gas shift reaction. Catalysts. 2017. https://doi.org/10.3390/catal7020048.
Si R, Raitano J, Yi N, Zhang L, Chan SW, Flytzani-Stephanopoulos M. Structure sensitivity of the low-temperature water-gas shift reaction on Cu–CeO2 catalysts. Catal Today. 2012. https://doi.org/10.1016/j.cattod.2011.09.008.
Duplančić M, Kurajica S, Tomašić V, Minga I. Catalytic oxidation of toluene on hydrothermally prepared ceria nanocrystals. Chem Biochem Eng Q. 2017;31:375–83. https://doi.org/10.15255/CABEQ.2017.1098.
Aranda A, Agouram S, López JM, Mastral AM, Sellick DR, Solsona B, Taylor SH, García T. Oxygen defects: the key parameter controlling the activity and selectivity of mesoporous copper-doped ceria for the total oxidation of naphthalene. Appl Catal B. 2012. https://doi.org/10.1016/j.apcatb.2012.07.033.
Kastrinaki G, Lorentzou S, Konstandopoulos AG. Soot oxidation kinetics of different ceria nanoparticle catalysts. Emiss Control Sci and Technol. 2015. https://doi.org/10.1007/s40825-015-0021-z.
Machida M, Murata Y, Kishikawa K, Zhang D, Ikeue K. On the reasons for high activity of CeO2 catalyst for soot oxidation. Chem Mater. 2008. https://doi.org/10.1021/cm800832w.
Miceli P, Bensaid S, Russo N, Fino D. CeO2-based catalysts with engineered morphologies for soot oxidation to enhance soot-catalyst contact. Nanoscale Res Lett. 2014. https://doi.org/10.1186/1556-276x-9-254.
Lin F, Wu X, Liu S, Weng D, Huang Y. Preparation of MnOx–CeO2–Al2O3 mixed oxides for NOx-assisted soot oxidation: activity, structure and thermal stability. Chem Eng J. 2013. https://doi.org/10.1016/j.cej.2013.04.006.
Melchionna M, Fornasiero P. The role of ceria-based nanostructured materials in energy applications. Mater Today. 2014. https://doi.org/10.1016/j.mattod.2014.05.005.
Liu S, Wu X, Weng D, Ran R. Ceria-based catalysts for soot oxidation: a review. J Rare Earths. 2015. https://doi.org/10.1016/S1002-0721(14)60457-9.
Venâncio SA, De Miranda PEV. Synthesis of CeAlO3/CeO2–Al2O3 for use as a solid oxide fuel cell functional anode material. Ceram Int. 2011. https://doi.org/10.1016/j.ceramint.2011.05.054.
Ramakrishnan G, Naveen K. Emission and dynamic characteristics of three way catalytic converter by computational fluid dynamics. Int J Eng Sci. 2016;6:3503–10.
Chen HY, Chang HL. Development of low temperature three-way catalysts for future fuel efficient vehicles. Johnson Matthey Technol Rev. 2015. https://doi.org/10.1595/205651315X686011.
Kundakovic L, Flytzani-Stephanopoulos M. Cu- and Ag-modified cerium oxide catalysts for methane oxidation. J Catal. 1998;179:203–11. https://doi.org/10.1006/jcat.1998.2213.
Dziembaj R, Molenda M, Chmielarz L, Zaitz MM, Piwowarska Z, Rafalska-Lasocha A. Optimization of Cu doped ceria nanoparticles as catalysts for low-temperature methanol and ethylene total oxidation. Catal Today. 2011. https://doi.org/10.1016/j.cattod.2010.11.061.
Heo I, Schmieg SJ, Oh SH, Li W, Peden CHF, Kim CH, Szanyi J. Improved thermal stability of a copper-containing ceria-based catalyst for low temperature CO oxidation under simulated diesel exhaust conditions. Catal Sci Technol. 2018. https://doi.org/10.1039/C7CY02288C.
Rupp JLM, Infortuna A, Gauckler LJ. Microstrain and self-limited grain growth in nanocrystalline ceria ceramics. Acta Mater. 2006. https://doi.org/10.1016/j.actamat.2005.11.032.
Kurajica S, Mužina K, Dražić G, Matijašić G, Duplančić M, Mandić V, Župančić M, Munda IK. A comparative study of hydrothermally derived Mn, Fe Co, Ni, Cu and Zn doped ceria nanocatalysts. Mater Chem Phys. 2020. https://doi.org/10.1016/j.matchemphys.2020.122689.
Li S, Wang N, Yue Y, Wang G, Zu Z, Zhang Y. Copper doped ceria porous nanostructures towards a highly efficient bifunctional catalyst for carbon monoxide and nitric oxide elimination. Chem Sci. 2015. https://doi.org/10.1039/C5SC00129C.
Zhou L, Li X, Yao Z, Chen Z, Hong M, Zhu R, Liang Y, Zhao J. Transition-metal doped ceria microspheres with nanoporous structures for CO oxidation. Sci Rep. 2016. https://doi.org/10.1038/srep23900.
Kurajica S, Munda IK, Dražić G, Mandić V, Mužina K, Bauer L, Matijašić G. Manganese-doped, hydrothermally-derived ceria: the occurrence of birnessite and the distribution of manganese. Ceram Int. 2020. https://doi.org/10.1016/j.ceramint.2020.05.025.
Mužina K, Kurajica S, Dražić G, Guggenberger P, Matijašić G. True doping levels in hydrothermally derived copper-doped ceria. J Nanopart Res. 2021. https://doi.org/10.1007/s11051-021-05274-6.
Kurajica S, Munda IK, Brleković F, Mužina K, Dražić G, Šipušić J, Mihaljević M. Manganese-doped ceria nanoparticles grain growth kinetics. J Solid State Chem. 2020. https://doi.org/10.1016/j.jssc.2020.121600.
Kurajica S, Minga I, Guliš M, Mandić V, Simčić I. High surface area ceria nanoparticles via hydrothermal synthesis experimental design. J Nanomater. 2016. https://doi.org/10.1155/2016/7274949.
Chen PL, Chen IW. Grain growth in CeO2: dopant effects, defect mechanism, and solute drag. J Am Ceram Soc. 1996. https://doi.org/10.1111/j.1151-2916.1996.tb07997.x.
Lopez HF, Mendoza H. Temperature effects on the crystallization and coarsening of nano-CeO2 powders. Nanomaterials. 2013. https://doi.org/10.1155/2013/208614.
Ivanov VK, Polezhaeva OS, Kopitsa GP, Fedorov PP, Pranzas K, Runov VV. Specifics of high-temperature coarsening of ceria nanoparticles. Russ J Inorg Chem. 2009. https://doi.org/10.1134/S0036023609110023.
Liang H, Raitano JM, He G, Akey AJ, Herman IP, Zhang L, Chan SW. Aqueous co-precipitation of Pd-doped cerium oxide nanoparticles: chemistry, structure, and particle growth. J Mater Sci. 2012. https://doi.org/10.1007/s10853-011-5798-8.
Li JG, Ikegami T, Wang Y, Mori T. Nanocrystalline Ce1-xYxO2-x/2 (0<x<0.35) oxides via carbonate precipitation: synthesis and characterization. J Solid State Chem. 2002. https://doi.org/10.1006/jssc.2002.9678.
Klug HP, Alexander LE. X-Ray diffraction procedures. 2nd ed. New York: Wiley; 1974.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012. https://doi.org/10.1038/nmeth.2089.
Lifshitz IM, Slyozov VV. The kinetics of precipitation from supersaturated solid solutions. J Phys Chem Solid. 1961. https://doi.org/10.1016/0022-3697(61)90054-3.
Wagner C. Theorie der Alterung von Niederschlaegen durch Umloesen (Ostwaldreifung). Z Elektro. 1961. https://doi.org/10.1002/bbpc.19610650704.
Zgalat-Lozynskyy O, Ragulya A. Densification kinetics and structural evolution during microwave and pressureless sintering of 15 nm titanium nitride powder. Nanoscale Res Lett. 2016. https://doi.org/10.1186/s11671-016-1316-x.
Avgouropoulos G, Ioannides T, Matralis H. Influence of the preparation method on the performance of CuO–CeO2 catalysts for the selective oxidation of CO. Appl Catal B. 2005. https://doi.org/10.1016/j.apcatb.2004.07.017.
Liu W, Flytzani-Stephanopoulos M. Total oxidation of carbon monoxide and methane over transition metal fluorite oxide composite catalysts: I catalyst composition and activity. J Catal. 1995. https://doi.org/10.1006/jcat.1995.1132.
Bera P, Priolkar KR, Sarode PR, Hegde MS, Emura S, Kumashiro R, Lalla NP. Structural investigation of combustion synthesized Cu/CeO2 catalysts by EXAFS and other physical techniques: formation of a Ce1-xCuxO2-δ solid solution. Chem Mater. 2002;14(8):3591–601.
Ni DW, Schmidt CG, Teocoli F, Kaiser A, Bøhm-Andersen K, Ramousse S, Esposito V. Densification and grain growth during sintering of porous Ce0.9Gd0.1O1.95 tape cast layers: a comprehensive study on heuristic methods. J Eur Ceram Soc. 2013;33:2529–37. https://doi.org/10.1016/j.jeurceramsoc.2013.03.025.
Wynblatt P, Gjostein NA. Particle growth in model supported metal catalysts-I Theory. Acta Metall. 1976. https://doi.org/10.1016/0001-6160(76)90034-1.
Wang Y, Mori T, Li JG, Ikegami T. Low-temperature synthesis of praseodymium-doped ceria nanopowders. J Am Ceram Soc. 2002. https://doi.org/10.1111/j.1151-2916.2002.tb00591.x.
Banfield JF, Navrotsky A. Nanoparticles and the environment. Rev Mineral Geochem. 2001. https://doi.org/10.2138/rmg.2001.44.01.
Ren R, Wu YC, Tang WM, Wang FT, Wang TG, Zheng ZX. Synthesis and grain growth kinetics of in-situ FeAl matrix nanocomposites (II): structural evolution and grain growth kinetics of mechanically alloyed Fe-Al-Ti-B composite powder during heat treatment. Trans Nonferrous Metals Soc. 2008. https://doi.org/10.1016/S1003-6326(08)60012-6.
Acknowledgements
The sustenance of the University of Zagreb is gratefully acknowledged.
Funding
This work has been fully supported by Croatian Science Foundation under the project IP-01-2018-2963.
Author information
Authors and Affiliations
Contributions
K.M. and S.K. involved in conceptualization; K.M., S.K., and F.B. contributed to methodology; K.M., S.K., F.B., D.J., G.D., L.V., and H.B. contributed to formal analysis and investigation; K.M. involved in writing—original draft preparation; K.M., S.K., F.B., D.J., and G.D. involved in writing—review and editing; K.M., F.B., and G.D. involved in visualization; S.K. involved in supervision; S.K. involved in project administration; S.K. contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mužina, K., Kurajica, S., Brleković, F. et al. Thermal stability study of hydrothermally derived copper-doped cerium (IV) oxide nanoparticles. J Therm Anal Calorim 148, 1657–1667 (2023). https://doi.org/10.1007/s10973-022-11375-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11375-8