Abstract
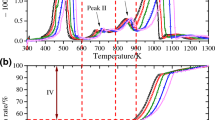
Gas chromatography (GC), ion chromatography (IC), and gas chromatography–mass spectrometry were used to investigate the thermal degradation of hexafluoropropane (HFC-236fa) fire suppression agent between 500 and 850 °C (GC–MS). The results show that the pyrolysis characteristics of HFC-236fa can only occur when the temperature is above 600 °C. The gas products of HFC-236fa pyrolysis mainly contain CHF3, CF2 = CHF, CF3C ≡ CCF3, and (CF3)2C = CF2. The concentration of each component is different with different pyrolysis temperatures and residence times. Moreover, the possible decomposition mechanism of HFC-236fa was proposed based on density functional theory, which was achieved via obtaining the activation energy and the details of dissociation pathways. By under the experimental studies and theoretical calculation, the pyrolysis of HFC-236fa was investigated.






Similar content being viewed by others
References
Lovelock JE. Atmospheric halocarbons and stratospheric ozone. Nature. 1974;252(5481):292–4. https://doi.org/10.1038/252292a0.
Safiuddin M, Sarbatly R. Global ozone depletion: causes, effects and preventive measures. Borneo Sci. 2001;10:73–89.
DiNenno PJ, Harrington JL. Halon alternative protection options. The Report on Proposals for the 1999 Spring Meedng. 2001;580–614.
Rubenstein R. Human health and environmental toxicity issues for evaluation of halon replacements. Toxicol Lett. 1993;68(1–2):21–4. https://doi.org/10.1016/0378-4274(93)90114-D.
Wallington TJ, Schneider WF, Worsnop DR, et al. The environmental impact of CFC replacements HFCs and HCFCs. Environ Sci Technol. 1994;28(7):320A-A326. https://doi.org/10.1021/es00056a714.
Tsai WT. An overview of environmental hazards and exposure risk of hydrofluorocarbons (HFCs). Chemosphere. 2005;61(11):1539–47. https://doi.org/10.1016/j.chemosphere.2005.03.084.
Powell RL. CFC phase-out: Have we met the challenge? J Fluorine Chem. 2002;114(2):237–50. https://doi.org/10.1016/S0022-1139(02)00030-1.
Trochimowicz HJ. Industrial research on alternative fluorocarbons. Toxicol Lett. 1993;68(1):25–30. https://doi.org/10.1016/0378-4274(93)90115-E
Nappa MJ, Sievert AC. Process for the manufacture of 1, 1, 1, 3, 3, 3,-hexafluoropropane.U.S. Patent 5,414,165. 1995.
Vinegar A, Buttler GW, Caracci MC et al. Gas Uptake Kinetics of 1, 1, 1, 3, 3, 3-Hexafluoropropane (HFC-236fa) and Identification of its Potential Metabolites. Geo-Centers Inc Newton Centre MA,1995.
Rao V, Sievert A, Miller R. Noncatalytic manufacture of 1, 1, 3, 3, 3-pentafluoropropene from 1, 1, 1, 3, 3, 3-hexafluoropropane. U.S. Patent 11/259,901.2006.
Vollmer MK, Miller BR, Rigby M, et al. Atmospheric histories and global emissions of the anthropogenic hydrofluorocarbons HFC-365mfc, HFC-245fa, HFC-227ea, and HFC-236fa. J Geophys Res. 2011. https://doi.org/10.1029/2010JD015309.
Borghhetti L, Chaer S, Ferguson D, et al. Options for aircraft engine fire protection. WA: International aircraft system fire protection working group, 2000.
Copeland G, Lee EPF, Dyke JM, et al. Study of pentafluoroethane and its thermal decomposition using UV photoelectron spectroscopy and ab initio molecular orbital calculations. J Phys Chem A. 2010;114(4):1816–25. https://doi.org/10.1021/jp909681s.
Takahashi K, Harada A, Horigome S, et al. Thermal decompositons of 1, 1, 1-trifluoroethane and pentafluoroethane in a turbulent flow reactor. Combust Sci Technol. 2007;179(7):1417–32. https://doi.org/10.1080/00102200601181962.
Zhou X, Chen W, Chao M, et al. The study of thermal decomposition of 2-bromo-3, 3, 3-trifluoropropene and its fire-extinguishing mechanism. J Fluorine Chem. 2013;153:101–6. https://doi.org/10.1016/j.jfluchem.2013.05.008.
Zhou X, Lu D, Chao M, et al. Experimental and theoretical studies on the thermal decomposition of 1, 1, 2, 2, 3, 3, 4-heptafluorocyclopentane. J Fluorine Chem. 2014;164:70–7. https://doi.org/10.1016/j.jfluchem.2014.04.007.
Angelino G, Invernizzi C. Experimental investigation on the thermal stability of some new zero ODP refrigerants. Int J Refrig. 2003;26(1):51–8. https://doi.org/10.1016/S0140-7007(02)00023-3.
Zhou XM, Zhou B, Chen T, et al. Pyrolysis process of 1, 1, 1, 3, 3, 3-Hexafluoropropane and its fire suppression mechanism. J Combust Sci Technol. 2011;5:05.
He B, Lei B, Zhao C, et al. Experimental study on the effects of different ratios of inert gas and hexafluoropropane (HFC-236fa) on coal combustion. Energy Sour Part A Recov Util Environ Effects. 2020. https://doi.org/10.1080/15567036.2020.1804015.
Garland NL, Nelson HH. Temperature dependent kinetics of the reaction OH+ CF3CH2CF3. 1996;248(3–4): 296–300. https://doi.org/10.1016/0009-2614(95)01288-5.
Gao H, Wang Y, Wan SQ, et al. Theoretical investigation of the hydrogen abstraction from CF3CH2CF3 by OH radicals, F, and Cl atoms: a dual-level direct dynamics study. J Mol Struct THEOCHEM. 2009. https://doi.org/10.1016/j.theochem.2009.07.024.
Wang T, Hu Y, Zhang P, et al. Study on thermal decomposition properties and its decomposition mechanism of pentafluoroethane (HFC-125) fire extinguishing agent. J Fluorine Chem. 2016;190:48–55. https://doi.org/10.1016/j.jfluchem.2016.08.006.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77(18):3865. https://doi.org/10.1103/PhysRevLett.77.3865.
Delley B. From molecules to solids with the DMol3 approach. The J Chem Phys. 2000;113(18):7756–64. https://doi.org/10.1063/1.1316015.
Delley B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys. 1990;92(1):508–17. https://doi.org/10.1063/1.458452.
Dolg M, Wedig U, Stoll H, et al. Energy-adjusted abinitio pseudopotentials for the first row transition elements. J Chem Phys. 1987;86(2):866–72. https://doi.org/10.1063/1.452288.
Bergner A, Dolg M, Küchle W, et al. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol Phys. 1993;80(6):1431–41. https://doi.org/10.1080/00268979300103121.
Henkelman G, Jónsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys. 2000;113(22):9978–85. https://doi.org/10.1063/1.1323224.
Halgren TA, Lipscomb WN. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett. 1977;49(2):225–32. https://doi.org/10.1016/0009-2614(77)80574-5.
Acknowledgements
The work was supported by the Scientific Research Project of Jiangsu Police Institute of China (Grant No. 2017SJYZZ04), Key Laboratory of Fire & Rescue Technology and Equipment,Ministry of Emergency Management (Grant No. 2020XFZB06), the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the collaborative Innovation Center of Social Safety Science and Technology. The authors are also appreciated for the full support from Pine Ridge Laboratory of Advanced Materials, Sichuan Easy Scientific Research Community Technology Co., Ltd., Mianyang 621050, Sichuan, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, T., Zhang, P. & Pan, Rm. Research on the pyrolysis of hexafluoropropane (HFC-236fa) fire extinguishing agent and decomposition mechanism. J Therm Anal Calorim 147, 11591–11599 (2022). https://doi.org/10.1007/s10973-022-11331-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11331-6