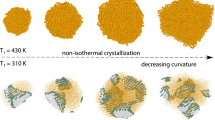
Abstract
Since 1998, the NPK method developed by R. Serra, J. Sempere and R. Nomen is used by many research groups to simulate and even sometimes to better understand the thermal behavior of many chemical reactions. The NPK method has never been used for crystallization processes, nor pure products or polymers. It is well known that the degree of crystallization of a polymer is a function of its processing and has crucial consequences on its final properties, so a considerable number of papers have been published to describe this phenomenon. Between them, we can find the description of phenomenological models like the ones of Lauritzen–Hoffman, Avrami, Šesták–Berggren or Sbirrazzuoli or functional methods like the model-free method of Vyazovkin. Our work aims to study the applicability of the NPK method to a polymer crystallization phenomenon from thermal analytical data and to verify that the information obtained agrees with the most accepted models. This contribution shows the first results working with isotactic polypropylene crystallization during the cooling down of melt material.





Similar content being viewed by others
References
Mileva D, Tranchida D, Gahleitner M. Designing polymer crystallinity: an industrial perspective. Polym Cryst. 2018;1: e10009. https://doi.org/10.1002/pcr2.10009.
Li J, Zhou C, Wang G, Tao Y, Liu Q, Li Y. Isothermal and nonisothermal crystallization kinetics of elastomeric polypropylene. Polym Test. 2002;21:583–9.
Crompton TR. Polymer reference book. 1st ed. London: iSmithers Rapra Publishing; 2006.
Menczel JD, Judovits L, Prime RB, Bair HE, Reading M, Swier S. Differential scanning calorimetry (DSC). In: Menczel JD, Prime RB, editors. Thermal analysis of polymers: fundamentals and applications. Hoboken: Wiley; 2009. p. 7–239.
Baltá Calleja FJ, Ezquerra TA. Polymer Crystallization: General Concepts of Theory and Experiment. 2001. https://doi.org/10.1016/B0-08-043152-6/01289-4.
Zhang MC, Guo B, Xu J. A review on polymer crystallization theories. Curr Comput-Aided Drug Des. 2017;7:4.
Serra R, Nomen R, Sempere J. The non-parametric kinetics a new method for the kinetic study of thermoanalytical data. J Therm Anal Calorim. 1998;52:933–43.
Vyazovkin S, Stone J, Sbirrazzuoli N. Hoffman–Lauritzen parameters for non-isothermal crystallization of poly (ethylene terephthalate) and poly (ethylene oxide) melts. J Therm Anal Calorim. 2005;80:177–80.
Tomellini M, Fanfoni M. Beyond the constraints underlying Kolmogorov-Johnson-Mehl-Avrami theory related to the growth laws. Phys Rev E. 2012;85:021606.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S. Is the Kissinger equation applicable to the processes that occur on cooling? Macromol Rapid Commun. 2002;23:771–5.
Ferrer N, Serra E, Sempere J, Nomen R. Non-parametric kinetic analysis of autocatalytic reactions. J Loss Prev Process Ind. 2017;49:357–66.
Sempere J, Nomen R, Serra E, Sempere B. Nonparametric kinetic methods. In: Anonymous thermal analysis of micro, nano-and non-crystalline materials. Springer, 2012. pp. 351–369.
Polschikov SV, Nedorezova PM, Monakhova TV, Klyamkina AN, Shchegolikhin AN, Krasheninnikov VG, Muradyan VE, Popov AA, Margolin AL. Composite materials based on fullerenes C 60/C 70 and polypropylene prepared via in situ polymerization. Polym Sci Ser B. 2013;55:286–93.
Nam B, Park OO, Kim S. Properties of isotactic polypropylene/atactic polypropylene blends. Macromol Res. 2015;23:809–13.
Vyazovkin S. Activation energies and temperature dependencies of the rates of crystallization and melting of polymers. Polymers. 2020;12:1070.
Blaine RL. Isothermal crystallization of polypropylene by differential scanning calorimetry. Am Lab. 2002;34:18–23.
Zhu X, Yan D. Influence of the order of polymer melt on the crystallization behavior: II. Crystallization kinetics of isotactic polypropylene. Colloid Polym Sci. 2001;279:546–53.
Huang J. Dispersion, crystallization kinetics, and parameters of Hoffman–Lauritzen theory of polypropylene and nanoscale calcium carbonate composite. Polym Eng Sci. 2009;49:1855–64.
Qiu S, Zheng Y, Zeng A, Guo Y. Prediction of non-isothermal crystallization parameters for isotactic polypropylene. Thermochim Acta. 2011;512:28–33.
Yamada K, Hikosaka M, Toda A, Yamazaki S, Tagashira K. Equilibrium melting temperature of isotactic polypropylene with high tacticity: 1. Determination by differential scanning calorimetry. Macromolecules. 2003;36:4790–801.
Wunderlich B. The ATHAS database on heat capacities of polymers. 1995;67:1019–26.
Razavi-Nouri M, Ghorbanzadeh-Ahangari M, Fereidoon A, Jahanshahi M. Effect of carbon nanotubes content on crystallization kinetics and morphology of polypropylene. Polym Test. 2009;28:46–52.
Vyazovkin S, Sbirrazzuoli N. Isoconversional approach to evaluating the Hoffman–Lauritzen parameters (U* and Kg) from the overall rates of nonisothermal crystallization. Macromol Rapid Commun. 2004;25:733–8.
Acknowledgements
The authors are grateful to Prof. Carlos Juárez, M.Sc. Violeta Chichique and to the students Oscar Carías, Albert Puigdellibol, César García and Wendy Torres for their collaboration in this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Frida Monzón. The first draft of the manuscript was written by all authors, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monzón, F., Rovira, M.D., Sempere, J. et al. Determination of polymer crystallization kinetics with the NPK method. J Therm Anal Calorim 147, 10089–10093 (2022). https://doi.org/10.1007/s10973-022-11320-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11320-9