Abstract
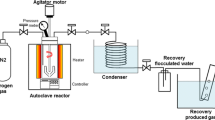
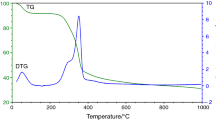
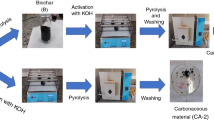
Slow pyrolysis and gasification reactivities of waste biochar samples, produced after hydrothermal treatment of spent coffee grounds, were determined using a thermogravimetric analyser (TGA) at 900 °C under CO2. Biochar samples were prepared by wet oxidation treatment in 30 vol.% hydrogen peroxide at room temperature for 2, 6, and 24 h and at 80 °C for 0.5, 1, and 2 h. NMR results showed that the samples treated at 80 °C showed a slight decrease in the amount of aromatic carbons and a significant reduction in the number of aliphatic carbons. The wet oxidation-treated biochar was thermally treated at 900 °C (10 °C min−1) under N2 for 2 h to produce a coke substitute. Results showed that the wet oxidation treatment of the samples at room temperature did not show much degradation, but samples treated at 80 °C showed higher mass losses. The temperature at which wet oxidation is performed has a significant effect during the process. The pyrolysis chars were investigated for their CO2 or gasification reactivities. CO2 isothermal gasification was used to determine the reactivities of the produced substitute coke char samples and industrial coke sample. The prepared substitute coke chars and the industrial coke samples were subjected to thermogravimetric analyses at an isothermal temperature of 900 °C in a CO2 atmosphere. Characterization of the prepared substituted coke samples was carried out using nuclear magnetic resonance (NMR). The CO2 gasification results showed that the average initial reactivities, the reactivities at 50% conversion, and the average final reactivities of the prepared samples were up to three times that of the industrial coke sample. The pyrolysis char of the coke substitute sample prepared for 2 h at 80 °C with a wet oxidation method has an average initial reactivity of 6.59 × 10–3 min−1, reactivity at 50% conversion of 15.73 × 10–3 min−1, and 9.6 × 10–3 min−1 for average final reactivity. The coke substitute samples produced from the waste coffee ground biochar subjected to a wet oxidation treatment method may be utilized as a coke substitute, with similar or higher gasification reactivity than industrial coke.





Similar content being viewed by others
References
Rodero JI, Sancho-Gorostiaga J, Ordiales M, Fernández-González D, Mochón J, Ruiz-Bustinza I, Fuentes A, Verdeja ILF. Blast furnace and metallurgical coke’s reactivity and its determination by thermal gravimetric analysis. Ironmak Steelmak. 2015;42:618–25.
Hilding T, Gupta S, Sahajwalla V, Björkman B, Wikström JO. Degradation behaviour of a high CSR coke in an experimental blast furnace: effect of carbon structure and alkali reactions. ISIJ Int. 2005;45:1041–50.
Menendez JA, Alvarez R, Pis JJ. Determination of metallurgical coke reactivity at INCAR: NSC and ECE-INCAR reactivity tests. Ironmak Steelmak. 1999;26:117–21.
Díaz-Faes E, Barriocanal C, Diez MA, Alvarez R. Applying TGA parameters in coke quality prediction models. J Anal Appl Pyrolysis. 2007;79:154–60.
Alvarez R, Diez MA, Barriocanal C, Diaz-Faes E, Cimadevilla JLG. An approach to blast furnace coke quality prediction. Fuel. 2007;86:2159–66.
Dıez MA, Alvarez R, Barriocanal C. Coal for metallurgical coke production: predictions of coke quality and future requirements for coke making. Int J Coal Geo. 2002;50:389–412.
Patrick J. The report. Coke quality and its prediction, Commission of the European Communities, Directorate-General Energy (1990). http://publications.europa.eu/resource/cellar/15c1e068-4039-4182-87c4-20c079f0798d.0001.02/DOC_1
Loison R, Foch P, Boyer A. Coke: quality and production (2014), Elsevier. https://books.google.co.za/books?hl=en&lr=&id=WIyjBQAAQBAJ&oi=fnd&pg=PP1&dq=Coke:+quality+and+production&ots=E8OpPYXn6K&sig=md2uE7QVf9cDvRhGUY5qJNLfsnY&redir_esc=y#v=onepage&q=Coke%3A%20quality%20and%20production&f=false
Zhang Q, Wu X, Feng A, Shi M. Prediction of coke quality at Baosteel. Fuel Process Technol. 2004;86:1–11.
Wang G, Zhang J, Hou X, Shao J, Geng W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour Technol. 2015;177(177):66–73.
Huo W, Zhou Z, Chen X, Dai Z, Yu G. Study on CO2 gasification reactivity and physical characteristics of biomass, petroleum coke and coal chars. Bioresour Technol. 2014;159:143–9.
Toor SS, Rosendahl LA, Hoffmann J, Pedersen TH, Nielsen RP, Søgaard ER. Hydrothermal liquefaction of biomass. In: Jin F, editor. Application of hydrothermal reactions to biomass conversion. Berlin: Springer; 2014. p. 189–217. https://doi.org/10.1007/978-3-642-54458-3_9.
Von Wielligh J, Schabort CJ, Venter R, Marx S. The evaluation of spent coffee grounds as feedstock for continuous hydrothermal liquefaction (2018). http://repository.nwu.ac.za/bitstream/handle/10394/34212/The_evaluation_of_spent_coffee_grounds.pdf?sequence=1&isAllowed=y
Tsai WT, Liu SC, Hsieh CH. Preparation and fuel properties of biochars from the pyrolysis of exhausted coffee residue. J Anal Appl Pyrolysis. 2012;93:63–7.
Cimadevilla JIG, Álvarez R, Pis JJ. Influence of coal forced oxidation on technological properties of cokes produced at laboratory scale. Fuel Process Technol. 2005;87:1–10.
Ashida R, Hashimoto A, Kawase M, Kubota Y. Production of metallurgical coke utilizing low-rank coal depolymerized by wet oxidation. ISIJ Int. 2019;59:1390–5.
Zubkova VV. The effect of coal charge density, heating velocity, and petroleum coke on the structure of cokes heated to 1800 °C. Fuel. 1999;78:1327–32.
Alvarez R, Pis JJ, Dıez MA, Barriocanal C, Canga CS, Menéndez JA. A semi-industrial scale study of petroleum coke as an additive in cokemaking. Fuel Process Technol. 1998;55:129–41.
Valia HS. 51st Ironmaking conference proceedings. Trans Iron Steel Soc AIME. 1992;51:443–7.
Pis JJ, Menendez JA, Parra JB, Alvarez R. Relation between texture and reactivity in metallurgical cokes obtained from coal using petroleum coke as additive. Fuel Process Technol. 2002;77:199–205.
Maree Z, Strydom CA, Bunt JR. Chemical and physical characterisation of spent coffee ground biochar treated by a wet oxidation method for the production of a coke substitute. J Waste Manag. 2020;113:422–9.
Everson RC, Okolo GN, Neomagus HW, Dos Santos JM. X-ray diffraction parameters and reaction rate modeling for gasification and combustion of chars derived from inertinite-rich coals. Fuel. 2013;109:148–56.
Everson RC, Okolo GN, Neomagus HW, Dos Santos JM. X-ray diffraction parameters and reaction rate modeling for gasification and combustion of chars derived from inertinite-rich coals. Fuel. 2013;109(109):148–56.
Yin Y, Zhang J, Sheng C. Effect of pyrolysis temperature on the char micro-structure and reactivity of NO reduction. Korean J Chem Eng. 2009;26:895–901.
Hu J, Shao J, Yang H, Lin G, Chen Y, Wang X, Zhang W, Chen H. Co-gasification of coal and biomass: synergy, characterization and reactivity of the residual char. Bioresour Technol. 2017;244:1–7.
Melkior T, Jacob S, Gerbaud G, Hediger S, Le Pape L, Bonnefois L, Bardet M. Co-gasification of coal and biomass: synergy, characterisation and reactivity of the residual char. Fuel. 2012;92:271–80.
Mafu LD, Neomagus HW, Everson RC, Okolo GN, Strydom CA, Bunt JR. The carbon dioxide gasification characteristics of biomass char samples and their effect on coal gasification reactivity during co-gasification. Bioresour Technol. 2018;258:70–8.
Cao X, Pignatello JJ, Li Y, Lattao C, Chappell MA, Chen N, Miller LF, Mao J. Characterisation of wood chars produced at different temperatures using advanced solid-state 13C NMR spectroscopic techniques. Energy Fuels. 2021;26:5983–91.
Mishra RK, Mohanty K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol. 2018;251:63–74.
Acknowledgements
The research presented is financially supported by the South African Research Chairs Initiative of the Department of Science and Technology (DST) and the National Research Foundation (NRF) of South Africa Coal Research Chair Grant No. 86880. Any opinion, conclusion, finding, or recommendation expressed in this material is that of the author(s), and the NRF does not accept any liability in this regard.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uwaoma, R.C., Maree, Z., Strydom, C.A. et al. Slow pyrolysis and gasification reactivity of thermally treated spent coffee grounds pre-treated by wet oxidation. J Therm Anal Calorim 147, 8613–8620 (2022). https://doi.org/10.1007/s10973-021-11118-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11118-1