Abstract
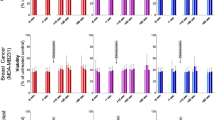
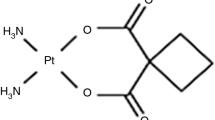
Breast cancers exhibit different response to drug treatment. In this work, we analyze and compare the effect of two anticancer drugs differing in their primary action, miltefosine and cisplatin (cis-Pt), on two different breast cancer (the low—(MCF-7) and high—(MDA-MB-231) metastatic) cell lines, and one normal epithelial (MCF-10A) breast cell lines. The effect of cip-Pt and miltefosine on the thermodynamic behavior of the cancer cell lines was analyzed by differential scanning calorimetry, the cell morphology and viability were determined by optical microscopy and MTT test. We revealed distinct effects of miltefosine and cis-Pt on the thermodynamic behavior and viability of the cancer and normal cells. Importantly, the normal MCF-10A cells were drastically affected by miltefosine, while not by cis-Pt. MDA-MB-231 cell line, on the other hand, is more susceptible to cis-Pt than MCF-7 cells, while both cancer cell lines are equally affected by miltefosine. The drug-associated alteration of the thermal unfolding of the cells constituents correlated with the changes in the cell viability. The altered thermodynamic behavior of the cancer cells upon the drug treatment strongly indicates altered conformations of the proteins in cancer cell membrane and cellular matrix, and the DNA-containing structures.






Similar content being viewed by others
References
DeSantis CE, Ma J, Sauer AG, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–48.
Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2017;13:215. https://doi.org/10.1186/bcr2889.
Dai X, Cheng H, Bai Zh, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8(16):3131–41.
LaPorta E, Welsh JE. Modeling vitamin D actions in triple negative/basal-like breast cancer. J Steroid Biochem Mol Biol. 2014;144A:65–73.
Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010;70:7970–80.
Konstantinov S, Berger M. Human urinary bladder carcinoma cell lines respond to treatment with alkylphosphocholines. Cancer Lett. 1999;144(2):153–60.
Berger MR, Tsoneva I, Konstantinov SM. Eibl H: Induction of apoptosis by erucylphospho-n, n, n-trimethylammonium is associated with changes in signal molecule expression and location. Ann N Y Acad Sci. 2003;1010:307–10.
Fieg M, Juergens M, Hiddemann W, Braess J. Cytotoxic activity of the third-generation bisphosphonate zoledronic acid in acute myeloid leukemia. Leukemia Res. 2007;31(4):531–9.
Pachioni J, Magalhães J, Lima EC, Bueno LM, Barbosa JF, de Sá MM, Rangel-Yagui CO. Alkylphospholipids: a promising class of chemotherapeutic agents with a broad pharmacological spectrum. J Pharm Pharm Sci. 2013;16(5):742–59.
Soto J, Soto P. Miltefosine: oral treatment of leishmaniasis. Expert Rev Anti Infect Ther. 2006;4(2):177–85.
Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67(11):2576–97.
Dineva I, Zaharieva M, Konstantinov S, Eibl H, Berger MR. Erufosine suppresses breast cancer in vitro and in vivo for its activity on PI3K, c-Raf and Akt proteins. J Cancer Res Clinical Oncol. 2012;138:1909–17.
Florea A, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–71. https://doi.org/10.3390/cancers3011351.
Dasari Sh, Tchounwou PB. Caisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;5:364–78.
Nishiyama N, Okazak S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, Nishio K, Matsumura Y, Kataoka K. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63:8977–83.
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–53.
Prabhakaran P, Hassiotou F, Blancafort P, Filgueira L. Cisplatin induces differentiation of breast cancer cells. Front Oncol. 2013. https://doi.org/10.3389/fonc.2013.00134.
Siddic ZH. Cisplatin mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–79.
Basu A, Krishnamurthy S. Cellular responses to cisplatin-induced DNA damage. J Nucleic Acids. 2010. https://doi.org/10.4061/2010/201367.
Nicolini C. Chromatin structure: from nuclei to genes. Anticancer Res. 1983;3(2):63–86.
Almagor M, Cole RD. Differential scanning calorimetry of nuclei as a test for the effects of anticancer drugs on human chromatin. Cancer Res. 1989;49(20):5561–6.
Lepock JR, Frey HE, Heynen ML, Senisterra GA, Warters RL. The nuclear matrix is a thermolabile cellular structure. Cell Stress Chaperon. 2001;6(2):136–47.
Todinova S, Stoyanova E, Krumova S, Iliev I, Taneva SG. Calorimetric signatures of human cancer cells and their nuclei. Thermochim Acta. 2016;623:95–101.
Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. NewYork: Cold Spring Harbor Laboratory Press; 2001.
Davido T, Getzenberg RH. Nuclear matrix proteins as cancer markers. J Cell Biochem. 2000;35:136–41.
Rynearson AL, Sussman CR. Nuclear structure, organization, and oncogenesis. J Gastroint Canc. 2011;42:112–7.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev. 2004;4:677–87.
True L, Jordan CD. The cancer nuclear microenvironment: Interface between light microscopic cytology and molecular phenotype. J Cell Biochem. 2008;104:1994–2003.
Lombardi ML, Zwerger M, Lammerding J. Biophysical assays to probe the mechanical properties of the interphase cell nucleus: substrate strain application and microneedle manipulation. J Vis Exp. 2011;55(e3087):1–6.
Rybczynska M, Liu R, Lu P, Sharom FJ, Steinfels E, Di Pietro A, Spitaler M, Grunicke H, Hofmann J. MDR1 causes resistance to the antitumour drug Miltefosine-. Br J Cancer. 2001;84:1405–11.
Barioni MB, Ramos AP, Zaniquelli MD, Acuna AU, Ito AS. Miltefozine and BODIPY-labeled alkylphosphocholine with leishmanicidalactivity: aggregation properties and characterization with model membranes. Biophys Chem. 2016;196:92–9.
Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Phys. 2013;4:185. https://doi.org/10.3389/fphys.2013.00185.
Petit K, Suwalsky M, Colina J, Aguilar L, Jemiola-Rzeminska M, Strzalka K. In vitro effects of antitumor drug miltefozine on human erythrocytes and molecular models of its membrane. BBA-Biomembr. 2019;1861:17–25.
Liang Sh-Sh, Wang T-N, Tsai E-M. Analysis of protein–protein interactions in mcf-7 and mda-mb-231 cell lines using phthalic acid chemical. Int J Mol Sci. 2014;15:20770–88.
Acknowledgements
This research was partly supported by COST COMULIS CA17121.
Funding
This work was supported by a grant from the National Science Fund of Bulgaria (Grant KP-05-COST/10 to B.N.) and by grant D01-392/2020 “National Center for Biomedical Photonics”, part of Bulgarian National Roadmap for Scientific Infrastructures 2020–2027, supported by Bulgarian Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Todinova, S., Nikolova, B., Iliev, I. et al. Thermodynamic behavior of breast cancer cell lines after miltefosine and cisplatin treatment. J Therm Anal Calorim 147, 7819–7828 (2022). https://doi.org/10.1007/s10973-021-11094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11094-6