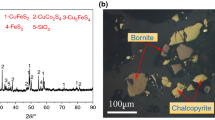
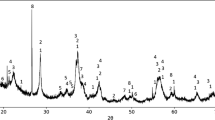
Abstract
Roasting is an essential process before ammonia leaching of low-grade rhodochrosite ore. Thermal decomposition characteristics and phase transformation rules of low-grade rhodochrosite ore with a particle size of less than 74 µm in N2, CO2 and air were studied. MnCO3, MgCO3 and CaCO3 in rhodochrosite are decomposed into MnO, MgO and CaO at 339 ℃, 306 ℃ and 842 ℃, respectively. MnO is oxidized to MnO2, Mn2O3 and Mn3O4 at 339 ℃, 533 ℃ and 1005 ℃, respectively. The TG/DTA and phase transformation results indicated that by thermal decomposition of rhodochrosite ore in air, N2 and CO2, MnCO3 is converted into Mn2O3, MnO and MnO, respectively. MnCO3 is converted to MnO at a higher temperature in a carbon dioxide atmosphere (512 ℃) than in a nitrogen atmosphere (400 ℃).










Similar content being viewed by others
References
Chen G, Li L, Tao C, Liu Z, Chen N, Peng J. Effects of microwave heating on microstructures and structure properties of the manganese ore. J Alloy Compd. 2016;657:515–8.
Kholmogorov A, Zhyzhaev A, Kononov U, Moiseeva G, Pashkov G. The production of manganese dioxide from manganese ores of some deposits of the Siberian region of Russia. Hydrometallurgy. 2000;56(1):1–11.
Xin B, Li T, Li X, Dan Z, Xu F, Duan N, et al. Reductive dissolution of manganese from manganese dioxide ore by autotrophic mixed culture under aerobic conditions. J Clean Prod. 2015;92:54–64.
Faria GLd, Tenório JAS, Jannotti N Jr, Araújo FdS. Disintegration on heating of a Brazilian manganese lump ore. Int J Miner Process. 2013;124:132–7.
Qing T, Zhong H, Shuai W, Li J, Liu G. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution. Trans Nonferrous Metals Soc China. 2014;24(3):861–7.
Figueira B, Angélica R, da Costa M, Pöllmann H, Schenzel K. Conversion of different Brazilian manganese ores and residues into birnessite-like phyllomanganate. Appl Clay Sci. 2013;86:54–8.
Yan W. Development of manganese ore resources and manganese slag industry in China. Abstr Chin Acad J. 2008;26:7–11.
Kanungo S, Mishra S. Dephosphorization of high-phosphorous manganese ores from Andhra Pradesh and southern Orissa, India, by roasting. Miner Metall Process. 2000;17(1):37–40.
Kanungo S, Sant B. Dephosphorization of P-rich Mn ores by selective leaching with dilute H2SO4. Proc Austral Inst Min Met. 1974;250:17–23.
Kanungo S, Mishra S. Reduction of phosphorus content of certain high phosphorus manganese ores of india by roasting with sodium chloride followed by leaching in acid medium. I: Statistical design of roasting experiments. Trans Indian Inst Metals. 2002;55(3):81–9.
Liu Y, Lin Q, Li L, Fu J, Zhu Z, Wang C, et al. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. Int J Min Sci Technol. 2014;24(4):567–71.
Arsent’ev V. Correlation between kinetic characteristic and surface effects manganese pulsed leaching in heterophase medium. Obogashch Rud. 1993;1–2:7–9.
Arsent’ev V, Kiselev K, Ryl’kov S, Sokolova V. Experimental processing of Vorkutinsk manganese ores by hydrometallurgical methods. Obogashchenie Rud. 1992;6:17–8.
Radmehr V, Koleini SMJ, Khalesi MR, Mohammadi MRT. Ammonia leaching: a new approach of copper industry in hydrometallurgical processes. J Inst Eng (India) Ser D. 2013;94(2):95–104.
Zhang W, Cheng CY. Manganese metallurgy review. Part I: leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy. 2007;89(3):137–59.
Liu B, Zhang Y, Su Z, Lu M, Peng Z, Li G, et al. Formation mechanism of MnxFe3−xO4 by solid-state reaction of MnO2 and Fe2O3 in air atmosphere: morphologies and properties evolution. Powder Technol. 2017;313:201–9.
El-Shobaky G, El-Barawy K. Effects of lithium doping on the thermal decomposition of manganese carbonate. Thermochim Acta. 1985;89:53–61.
El-Shobaky G, El-Barawy K, Ibrahim A. Thermal solid-solid interaction between potassium and manganese oxides. Thermochim Acta. 1986;102:21–7.
Vračar RŽ, Cerović KP. Manganese leaching in the FeS2–MnO2–O2–H2O system at high temperature in an autoclave. Hydrometallurgy. 2000;55(1):79–92.
Senanayake G. A mixed surface reaction kinetic model for the reductive leaching of manganese dioxide with acidic sulfur dioxide. Hydrometallurgy. 2004;73(3–4):215–24.
Su H, Wen Y, Wang F, Li X, Tong Z. Leaching of pyrolusite using molasses alcohol wastewater as a reductant. Miner Eng. 2009;22(2):207–9.
Song J, Zhu G, Zhao Y, Zhang P. Reduction treatment of low-grade manganese ore by biomass roasting. Acta Metall Sin (Engl Lett). 2010;23(3):223–9.
Zhang H, Zhu G, Yan H, Zhao Y, Li T, Feng X. Reduction of low-grade manganese dioxide ore pellets by biomass wheat stalk. Acta Metall Sin (Engl Lett). 2013;26(2):167–72.
Yang Z-p, Jin X-z, Zhu G-c. Study on the process of low grade manganese ore treated by ammonium calcination. China’s Mang Ind. 2006;3:12–4.
Jin X, Yang Z, Chen Z, Ma Y. Study on technology of low grade Manganese carbonate ore roasting by salt to enrich manganese. China’s Mang Ind. 2006;24:33–4.
Dollimore D, Tonge K. In HG Weidemann (Ed.), 3rd Int. Conf. Thermal Analysis Davois, 1971. Birkhaeuser, Basel; 1972.
Algoufi Y, Kabir G, Hameed B. Synthesis of glycerol carbonate from biodiesel by-product glycerol over calcined dolomite. J Taiwan Inst Chem Eng. 2017;70:179–87.
Nur ZS, Taufiq-Yap Y, Nizah MR, Teo SH, Syazwani O, Islam A. Production of biodiesel from palm oil using modified Malaysian natural dolomites. Energy Convers Manag. 2014;78:738–44.
Youssef N, Farid T, Selim M. Decomposition of hydrogen peroxide over pure and mixed copper oxide and manganese oxide prepared from carbonates. Commun Fac Sci Univ Ank Ser B. 1993;38:33–42.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tu, Z., Liang, X., Wu, C. et al. Thermal decomposition characteristics of low-grade rhodochrosite ore in N2, CO2 and air atmosphere. J Therm Anal Calorim 147, 6481–6488 (2022). https://doi.org/10.1007/s10973-021-10974-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10974-1