Abstract
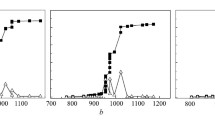
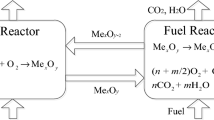
The oxidation characteristics of graphite with copper oxide nanoparticles as oxidizer mixed in stoichiometric proportion were studied using a thermogravimetric analyzer. Progress of reactions was followed by measuring the mass loss, evolved gas analysis, and energy-dispersive X-ray spectroscopy. The samples were heated from room temperature conditions to 975 °C at four different heating rates of 5, 10, 15, and 20 °C min -1. The exhaust gas composition was quantified using a non-dispersive infrared analyzer. Experimental results indicate that the system requires a critical CO concentration buildup before the combustion process accelerates. The carbon dioxide production begins and peaks later than carbon monoxide. A total of three peaks occur in both carbon monoxide and carbon dioxide evolution. An Arrhenius dependence for the peak separation times with respect to the starting peak or trough temperature was observed. This work experimentally identifies the various regimes of oxidation chemistry for this fuel oxidizer combination over the entire combustion process via the trajectory of CO and CO2 evolution with temperature. The paths in this trajectory are then mapped onto the mass loss profiles. Modulated TGA experiments were also conducted to obtain the activation energy during the combustion process that shows overlapping mass loss regions. This activation energy is obtained over a wide range of temperatures using this model free-approach. The activation energies obtained using the modulated TGA experiments were also compared to those obtained using the Flynn–Wall–Ozawa method. This work provides new insights into the oxidation kinetics of graphitic carbon with solid copper oxide as an oxidizer.















Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
IEA Coal. Analysis and forecasts to 2023. Paris: International Energy Agency; 2018.
Siriwardane R, Tian HJ, Richards G, Simonyi T, Poston J. Chemical-looping combustion of coal with metal oxide oxygen carriers. Energy Fuels. 2009;23(8):3885–92. https://doi.org/10.1021/ef9001605.
Sun XY, Xiang WG, Wang S, Tian WD, Xu X, Xu YJ, Xiao YH. Investigation of coal fueled chemical looping combustion using Fe3O4 as oxygen carrier: Influence of variables. J Therm Sci. 2010;19(3):266–75. https://doi.org/10.1007/s11630-010-0266-3.
Saha C, Bhattacharya S. Comparison of CuO and NiO as oxygen carrier in chemical looping combustion of a Victorian brown coal. Int J Hydrog Energy. 2011;36(18):12048–57. https://doi.org/10.1016/j.ijhydene.2011.06.065.
Wang BW, Xiao G, Song XY, Zhao HB, Zheng CG. Chemical looping combustion of high-sulfur coal with NiFe2O4-combined oxygen carrier. J Therm Anal Calorim. 2014;118(3):1593–602. https://doi.org/10.1007/s10973-014-4074-y.
Zhang S, Xiao R, Zheng WG. Comparative study between fluidized-bed and fixed-bed operation modes in pressurized chemical looping combustion of coal. Appl Energy. 2014;130:181–9. https://doi.org/10.1016/j.apenergy.2014.05.049.
Heydari M, Rahman M, Gupta R. Kinetic study and thermal decomposition behavior of lignite coal. Int J Chem Eng. 2015. https://doi.org/10.1155/2015/481739.
Rajendran S, Zhang S, Xiao R, Bhattacharya S. Use of synthetic oxygen carriers for chemical looping combustion of Victorian brown coal. Proc Combust Inst. 2015;35:3619–27. https://doi.org/10.1016/j.proci.2014.06.058.
Wang P, Means N, Shekhawat D, Berry D, Massoudi M. Chemical-looping combustion and gasification of coals and oxygen carrier development: a brief review. Energies. 2015;8(10):10605–35. https://doi.org/10.3390/en81010605.
Wang BW, Wang WS, Ma Q, Lu J, Zhao HB, Zheng CG. In-depth investigation of chemical looping combustion of a Chinese bituminous coal with CuFe2O4 combined oxygen carrier. Energy Fuels. 2016;30(3):2285–94. https://doi.org/10.1021/acs.energyfuels.5b02605.
Ishida M, Yamamoto M, Ohba T. Experimental results of chemical-looping combustion with NiO/NiAl2O4 particle circulation at 1200 degrees C. Energy Convers Manag. 2002;43(9–12):1469–78. https://doi.org/10.1016/S0196-8904(02)00029-8.
Noorman S, Gallucci F, Annaland MV, Kuipers H. Experimental investigation of a CuO/Al2O3 oxygen carrier for chemical-looping combustion. Ind Eng Chem Res. 2010;49(20):9720–8. https://doi.org/10.1021/ie100869t.
Fossdal A, Bakken E, Oye BA, Schoning C, Kaus I, Mokkelbost T, Larring Y. Study of inexpensive oxygen carriers for chemical looping combustion. Int J Greenh Gas Con. 2011;5(3):483–8. https://doi.org/10.1016/j.ijggc.2010.08.001.
Mendiara T, Abad A, De Diego LF, Garcia-Labiano F, Gayan P, Adanez J. Use of an Fe-based residue from alumina production as an oxygen carrier in chemical-looping combustion. Energy Fuels. 2012;26(2):1420–31. https://doi.org/10.1021/ef201458v.
Kuo YL, Hsu WM, Chiu PC, Tseng YH, Ku Y. Assessment of redox behavior of nickel ferrite as oxygen carriers for chemical looping process. Ceram Int. 2013;39(5):5459–65. https://doi.org/10.1016/j.ceramint.2012.12.055.
Fennell P. Calcium and chemical looping technology: an introduction. In: Fennell P, Anthony B, editors. Calcium and chemical looping technology for power generation and carbon dioxide (CO2) capture. Amsterdam: Woodhead Publishing; 2015. p. 3–14.
Hu WT, Donat F, Scott SA, Dennis JS. Kinetics of oxygen uncoupling of a copper based oxygen carrier. Appl Energy. 2016;161:92–100. https://doi.org/10.1016/j.apenergy.2015.10.006.
Guo L, Zhao HB, Zheng CG. Synthesis gas generation by chemical-looping reforming of biomass with natural copper ore as oxygen carrier. Waste Biomass Valoriz. 2015;6(1):81–9. https://doi.org/10.1007/s12649-014-9328-1.
Keattch CJ, Dollimore D. An introduction to thermogravimetry. 2nd ed. London: Heyden; 1969.
Brown WE, Dollimore D, Galwey AK. Reactions between inorganic solids. In: Bamford CH, Tipper CFH, editors. Reactions in the solid state. Amsterdam: Elsevier; 1980. p. 277.
Sohn HY, Szekely J. Reactions between solids through gaseous intermediates—I reactions controlled by chemical kinetics. Chem Eng Sci. 1973;28(10):1789–801. https://doi.org/10.1016/0009-2509(73)85061-4.
Blaine RL, Hahn BK. Obtaining kinetic parameters by modulated thermogravimetry. J Therm Anal. 1998;54:695–704. https://doi.org/10.1023/A:1010171315715.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett. 1966;4(5):323–8. https://doi.org/10.1002/pol.1966.11004050.
Blaine RL. Kinetic parameters of overlapping coal decomposition reactions by MTGA, TA instruments Report Number TA-253.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. https://doi.org/10.1246/bcsj.38.1881.
Brown WE, Dollimore D, Galwey AK. Theory of solid state reaction kinetics. In: Bamford CH, Tipper CFH, editors. Reactions in the solid state. Amsterdam: Elsevier; 1980. p. 99–109.
Venkatesh M, Ravi P, Tewari SP. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs Flynn–Wall–Ozawa Method. J Phys Chem A. 2013;117(40):10162–9. https://doi.org/10.1021/jp407526r.
Adánez-Rubio I, Gayán P, Abad A, García-Labiano F, De Diego LF, Adánez J. Kinetic analysis of a Cu-based oxygen carrier: relevance of temperature and oxygen partial pressure on reduction and oxidation reactions rates in chemical looping with oxygen uncoupling (CLOU). Chem Eng J. 2014;256:69–84. https://doi.org/10.1016/j.cej.2014.06.102.
Acknowledgements
Samuel Stuhlman acknowledges the assistantship support from the department of mechanical engineering at the University of Idaho. The energy-dispersive X-ray spectroscopy (EDS) was carried out at the electron microscopy center at the University of Idaho. We gratefully acknowledge the assistance of Dr. Thomas J. Williams with the EDS experiments.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Kamal Kumar contributed to the study conception and design. Samuel Stuhlman performed material preparation, data collection, and analysis. Samuel Stuhlman wrote the first draft of the manuscript, which was revised and edited by Kamal Kumar.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stuhlman, S., Kumar, K. Oxidation kinetics of graphite nanoparticles with copper oxide as oxygen carrier. J Therm Anal Calorim 147, 4165–4175 (2022). https://doi.org/10.1007/s10973-021-10796-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10796-1