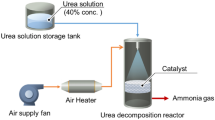
Abstract
In this paper, a new integrated system is proposed consisting of four subsystems, including a Cu–Cl cycle, a carbon capture cycle (sodium-based sorbent), an ammonia production unit, and a methanation unit. Carbon capturing cycle with sodium-based sorbent could adsorb 1.5 times more than other sorbents such as amine-based sorbent. Moreover, Cu–Cl cycle is one of the promising cycles in terms of economic and low temperature. Therefore, integrating these two cycles with ammonia and methane production unit is the novelty for this paper. Also, Aspen plus software was used to simulate the developed system to evaluate the process of the system. Moreover, sensitivity analysis and mass/energy balance are performed for the developed systems through the simulation. The energy required to capture carbon dioxide was found to be 6.313 MW per kg of CO2, and the overall efficiency of the system is equal to 19.6%.











Similar content being viewed by others
Abbreviations
- bar:
-
Pressure
- °C:
-
Degree Celsius
- Cp:
-
Specific heat (KJ mol−1 K−1)
- DHFORMa :
-
Standard heat of formation (kJ mol−1)
- DHSFRMb :
-
Standard solid heat of formation (kJ mol−1)
- DGFORMc :
-
Standard Gibbs free energy of formation (kJ mol−1)
- DGSFRMd :
-
Standard solid Gibbs free energy of formation (kJ mol−1)
- \(E_{{{\mathrm{CO}}_{2} }}\) :
-
Total energy consumption per one kg of CO2
- \(\eta_{{\mathrm{Cu - Cl}}}\) :
-
Energy efficiency of Cu–Cl cycle
- \(\eta_{{{\mathrm{sys}}}}\) :
-
Overall efficiency of the developed system
- \(\eta_{{{\mathrm{CCC}}}}\) :
-
Carbon capture cycle efficiency
- g:
-
Gram
- H:
-
Enthalpy of formation (kJ mol−1)
- h:
-
Hour
- J:
-
Joule
- K:
-
Kelvin
- k:
-
Kilo
- LHV:
-
Lower heat value (kJ kg−1)
- M:
-
Mega
- \(\dot{m}\) :
-
Mass flow (kg s−1)
- \(\dot{Q}\) :
-
Heat flow (kW)
- s:
-
Second
- V:
-
Electric voltage (v)
- W:
-
Watt (kJ s−1)
- \(\dot{W}\) :
-
Work (kW
- \(\Delta\) :
-
Delta
- \(\eta\) :
-
Efficiency
- aq:
-
Aqueous
- elec:
-
Electrical
- g:
-
Gas
- in:
-
Inlet
- l:
-
Liquid
- s:
-
Solid
- sys:
-
System
- CCC:
-
Carbon capture cycle
- CH4 :
-
Methane
- CO2 :
-
Carbon dioxide
- Cu:
-
Copper
- Cu–Cl:
-
Copper-chlorine cycle
- CuCl:
-
Copper monochloride
- CuCl2 :
-
Copper chloride
- Cu2OCl2 :
-
Copper oxychloride
- GHG:
-
Greenhouse gas
- H2 :
-
Hydrogen
- H2O:
-
Water
- HCl:
-
Hydrochloride acid
- HRSG:
-
Heat recovery steam generator
- O2 :
-
Oxygen
- N2 :
-
Nitrogen
- Na:
-
Sodium
- NH3 :
-
Ammonia
- NaOH:
-
Sodium hydroxide
- NaOHCO3 :
-
Sodium bicarbonate
- Na2CO3 :
-
Sodium carbonate
- Press:
-
Pressure
- SMR:
-
Steam methane reforming
- Temp:
-
Temperature
References
Ishaq H, Dincer I, Naterer GF. Industrial heat recovery from a steel furnace for the cogeneration of electricity and hydrogen with the copper-chlorine cycle. Energy Convers Manag. 2018;171(May):384–97. https://doi.org/10.1016/j.enconman.2018.05.039.
Valeh-e-Sheyda P, Rashidi H, Ghaderzadeh F. Integration of commercial CO2 capture plant with primary reformer stack of ammonia plant. J Therm Anal Calorim. 2019;135(3):1899–909. https://doi.org/10.1007/s10973-018-7215-x.
Orhan MF, Dincer I, Rosen MA. Thermodynamic analysis of the copper production step in a copper-chlorine cycle for hydrogen production. Thermochim Acta. 2008;480(1–2):22–9. https://doi.org/10.1016/j.tca.2008.09.014.
Environmental Protection Agency (EPA), “Nitrogen oxides (NOx), why and how they are controlled,” Epa-456/F-99–006R, no. November, p. 48, 1999, doi: EPA 456/F-99–006R.
Yao X, et al. The production of hydrogen through steam reforming of bio-oil model compounds recovering waste heat from blast furnace slag: Thermodynamic study. J Therm Anal Calorim. 2018;131(3):2951–62. https://doi.org/10.1007/s10973-017-6804-4.
Ratlamwala TAH, Dincer I. Energy and exergy analyses of a Cu–Cl cycle based integrated system for hydrogen production. Chem Eng Sci. 2012;84:564–73. https://doi.org/10.1016/j.ces.2012.08.052.
Barelli L, Bidini G, Gallorini F, Servili S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy. 2008;33(4):554–70. https://doi.org/10.1016/j.energy.2007.10.018.
Duan W, Yu Q. Thermodynamic analysis of hydrogen-enriched syngas generation coupled with in situ CO2 capture using chemical looping gasification method. J Therm Anal Calorim. 2018;131(2):1671–80. https://doi.org/10.1007/s10973-017-6596-6.
Huang CN, Shen HT. Maximum hydrogen production by using a gasifier based on the adaptive control design. Int J Hydrogen Energy. 2019;44(48):26248–60. https://doi.org/10.1016/j.ijhydene.2019.08.087.
Ge Z, Guo L, Jin H. Catalytic supercritical water gasification mechanism of coal. Int J Hydrogen Energy. 2020;45(16):9504–11. https://doi.org/10.1016/j.ijhydene.2020.01.245.
R. Guerrero-Lemus and J. M. Martıínez-Duart, Lecture Notes in Energy. 2012.
Naterer GF, et al. Clean hydrogen production with the Cu–Cl cycle-Progress of international consortium, II: Simulations, thermochemical data and materials. Int J Hydrogen Energy. 2011;36(24):15486–501. https://doi.org/10.1016/j.ijhydene.2011.08.013.
Naterer GF, Gabriel K, Wang ZL, Daggupati VN, Gravelsins R. Thermochemical hydrogen production with a copper-chlorine cycle. I: oxygen release from copper oxychloride decomposition. Int J Hydrogen Energy. 2008;33(20):5439–50. https://doi.org/10.1016/j.ijhydene.2008.05.035.
Ferrandon M, Daggupati V, Wang Z, Naterer G, Trevani L. Using XANES to obtain mechanistic information for the hydrolysis of CuCl2 and the decomposition of Cu2OCl2 in the thermochemical Cu–Cl cycle for H2 production. J Therm Anal Calorim. 2015;119(2):975–82. https://doi.org/10.1007/s10973-014-4240-2.
Lewis MA, Ferrandon MS, Tatterson DF, Mathias P. Evaluation of alternative thermochemical cycles - Part III further development of the Cu–Cl cycle. Int J Hydrogen Energy. 2009;34(9):4136–45. https://doi.org/10.1016/j.ijhydene.2008.09.025.
Wijayanta AT, Aziz M. Ammonia production from algae via integrated hydrothermal gasification, chemical looping, N2 production, and NH3 synthesis. Energy. 2019;174:331–8. https://doi.org/10.1016/j.energy.2019.02.190.
Gao W, et al. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers. Nat Energy. 2018;3(12):1067–75. https://doi.org/10.1038/s41560-018-0268-z.
Bagja F, Aziz M. ScienceDirect Integrated system of thermochemical cycle of ammonia, nitrogen production, and power generation. Int J Hydrogen Energy. 2019;44(33):17525–34. https://doi.org/10.1016/j.ijhydene.2019.05.110.
Di Carlo A, Dell’Era A, Del Prete Z. 3D simulation of hydrogen production by ammonia decomposition in a catalytic membrane reactor. Int J Hydrogen Energy. 2011;36(18):11815–24. https://doi.org/10.1016/j.ijhydene.2011.06.029.
Lan R, Irvine JTS, Tao S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int J Hydrogen Energy. 2012;37(2):1482–94. https://doi.org/10.1016/j.ijhydene.2011.10.004.
T. Chwo et al., “Pilot plant initial results for the methanation process using CO 2 from amine scrubbing at the Ł aziska power plant in Poland,” vol. 263, no. May 2019, 2020, doi: https://doi.org/10.1016/j.fuel.2019.116804.
Yoo M, Han SJ, Wee JH. Carbon dioxide capture capacity of sodium hydroxide aqueous solution. J Environ Manage. 2013;114:512–9. https://doi.org/10.1016/j.jenvman.2012.10.061.
P. Authors, J. Jones, C. Barton, M. Clayton, and A. Yablonsky, “SkyMine ® Carbon Mineralization Pilot Project Final Phase 1 Topical Report,” vol. 2011, no. September, 2011.
I. R. Salmón, R. Janssens, and P. Luis, “Mass and heat transfer study in osmotic membrane distillation- crystallization for CO 2 valorization as sodium carbonate,” vol. 176, pp. 173–183, 2017, doi: https://doi.org/10.1016/j.seppur.2016.12.010.
Tavan Y, Hosseini SH. A novel rate of the reaction between NaOH with CO2at low temperature in spray dryer. Petroleum. 2017;3(1):51–5. https://doi.org/10.1016/j.petlm.2016.11.006.
Berg RL, Vanderzee CE. Thermodynamics of carbon dioxide and carbonic acid: (a) the standard enthalpies of solution of Na2CO3(s), NaHCO3(s), and CO2(g) in water at 298.15 K; (b) the standard enthalpies of formation. J Chem Thermodyn. 1978;10(12):1113–36. https://doi.org/10.1016/0021-9614(78)90029-0.
Ishaq H, Dincer I. Exergy analysis and performance evaluation of a newly developed integrated energy system for quenchable generation. Energy. 2019;179:1191–204. https://doi.org/10.1016/j.energy.2019.05.050.
Ishaq H, Dincer I. Analysis and optimization for energy, cost and carbon emission of a solar driven steam-autothermal hybrid methane reforming for hydrogen, ammonia and power production. J Clean Prod. 2019;234:242–57. https://doi.org/10.1016/j.jclepro.2019.06.027.
Cai W, et al. Optimal sizing and location based on economic parameters for an off-grid application of a hybrid system with photovoltaic, battery and diesel technology. Energy. 2020;201:117480. https://doi.org/10.1016/j.energy.2020.117480.
J. Oh, D. Jung, S. Hwan, K. Roh, S. Ga, and J. H. Lee, “Design , simulation and feasibility study of a combined CO 2 mineralization and brackish water desalination process,” J. CO2 Util., vol. 34, no. July, pp. 446–464, 2019, doi: https://doi.org/10.1016/j.jcou.2019.07.004.
Ishaq H, Dincer I. A comparative evaluation of three Cu–Cl cycles for hydrogen production. Int J Hydrogen Energy. 2019;44(16):7958–68. https://doi.org/10.1016/j.ijhydene.2019.01.249.
Ishaq H, Dincer I. Investigation of an integrated system with industrial thermal management options for carbon emission reduction and hydrogen and ammonia production. Int J Hydrogen Energy. 2019;44(26):12971–82. https://doi.org/10.1016/j.ijhydene.2019.03.067.
A. Plus and U. Guide, “Aspen Plus ®.”
Nikulshina V, Ayesa N, Gálvez ME, Steinfeld A. Feasibility of Na-based thermochemical cycles for the capture of CO2 from air-Thermodynamic and thermogravimetric analyses. Chem Eng J. 2008;140(1–3):62–70. https://doi.org/10.1016/j.cej.2007.09.007.
Y. Liang, “Carbon Dioxide Capture From Flue Gas Using Regenerable Sodium-Based Sorbents,” pp. 1–137, 2003.
Liang Y, Harrison DP, Gupta RP, Green DA, McMichael WJ. Carbon dioxide capture using dry sodium-based sorbents. Energy Fuels. 2004;18(2):569–75. https://doi.org/10.1021/ef030158f.
Xie W, Chen X, Ma J, Liu D, Cai T, Wu Y. Energy analyses and process integration of coal-fired power plant with CO2 capture using sodium-based dry sorbents. Appl Energy. 2019. https://doi.org/10.1016/j.apenergy.2019.113434.
Zamfirescu C, Dincer I, Naterer GF. Thermophysical properties of copper compounds in copper-chlorine thermochemical water splitting cycles. Int J Hydrogen Energy. 2010;35(10):4839–52. https://doi.org/10.1016/j.ijhydene.2009.08.101.
Dinali MN, Dincer I. Development of a new trigenerational integrated system for dimethyl-ether, electricity and fresh water production. Energy Convers Manag. 2019;185(November 2018):850–65. https://doi.org/10.1016/j.enconman.2019.01.106.
Naterer GF, et al. Clean hydrogen production with the Cu–Cl cycle-Progress of international consortium, I: experimental unit operations. Int J Hydrogen Energy. 2011;36(24):15472–85. https://doi.org/10.1016/j.ijhydene.2011.08.012.
Baltrusaitis J. Sustainable ammonia production, vol. 5. Washington, DC: ACS Publications; 2017.
Lecker B, Illi L, Lemmer A, Oechsner H. Biological hydrogen methanation—a review. Bioresour Technol. 2017;245(August):1220–8. https://doi.org/10.1016/j.biortech.2017.08.176.
Baraj E, Vagaský S, Hlinčík T, Ciahotný K, Tekáč V. Reaction mechanisms of carbon dioxide methanation. Chem Pap. 2016;70(4):395–403. https://doi.org/10.1515/chempap-2015-0216.
Naterer GF, et al. Progress of international hydrogen production network for the thermochemical Cu–Cl cycle. Int J Hydrogen Energy. 2013;38(2):740–59. https://doi.org/10.1016/j.ijhydene.2012.10.023.
Avsec J, Wang Z, Naterer GF. Thermodynamic and transport properties of fluids and solids in a Cu–Cl solar hydrogen cycle. J Therm Anal Calorim. 2017;127(1):961–7. https://doi.org/10.1007/s10973-016-5875-y.
Ishaq H, Dincer I, Naterer GF. New trigeneration system integrated with desalination and industrial waste heat recovery for hydrogen production. Appl Therm Eng. 2018;142:767–78. https://doi.org/10.1016/j.applthermaleng.2018.07.019.
Wang W, Herreros JM, Tsolakis A, York APE. Ammonia as hydrogen carrier for transportation; investigation of the ammonia exhaust gas fuel reforming. Int J Hydrogen Energy. 2013;38(23):9907–17. https://doi.org/10.1016/j.ijhydene.2013.05.144.
Chaves IDG, López JRG, Zapata JLG, Robayo AL, Niño GR. Process analysis and simulation in chemical engineering. Process Anal Simul Chem Eng. 2015. https://doi.org/10.1007/978-3-319-14812-0.
Zhang G, Shi Y, Maleki A, Rosen MA. Optimal location and size of a grid-independent solar/hydrogen system for rural areas using an efficient heuristic approach. Renew Energy. 2020;156:1203–14. https://doi.org/10.1016/j.renene.2020.04.010.
Maleki A, Nazari MA, Pourfayaz F. Harmony search optimization for optimum sizing of hybrid solar schemes based on battery storage unit. Energy Rep. 2020. https://doi.org/10.1016/j.egyr.2020.03.014.
Ishaq H, Dincer I, Naterer GF. Exergy and cost analyses of waste heat recovery from furnace cement slag for clean hydrogen production. Energy. 2019;172:1243–53. https://doi.org/10.1016/j.energy.2019.02.026.
Daggupati VN, Naterer GF, Gabriel KS, Gravelsins RJ, Wang ZL. Equilibrium conversion in Cu–Cl cycle multiphase processes of hydrogen production. Thermochim Acta. 2009;496(1–2):117–23. https://doi.org/10.1016/j.tca.2009.07.009.
Science, E. Absorption of CO2 from modified flue gases of power generation Tarahan chemically using NaOH and Na2CO3 and biologically using microalgae
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izanloo, M., Mehrpooya, M. Investigation of a hybrid thermochemical Cu–Cl cycle, carbon capturing, and ammonia production process. J Therm Anal Calorim 144, 1907–1923 (2021). https://doi.org/10.1007/s10973-021-10768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10768-5