Abstract
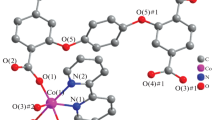
One cobalt (II) coordination polymer, named as [Co(3,3′-BDA)(H2O)2]n (3,3′-H2BDA is 3,3′-dicarboxyl-2,2′-bipyridine), has been synthesized under a simulated condition using an RD496-2000-type microcalorimeter. X-ray single-crystal structural analysis shows complex 1 is a one-dimensional chain-like structural feature and the same as the reported complex prepared under hydrothermal conditions. The hydrothermal processes of 1 were simulated by microcalorimetry in situ, and the different simulated keeping temperatures at 353.15 K, 393.15 K, 413.15 K, 433.15 K were adopted to verify the effect of temperature on the formation of 1. The results show that complex 1 has been formed when the reactants are mixed and reacted at room temperature, and the synthesis processes under different keeping temperatures contributed to the formation of its crystals. The solid-state reaction enthalpy change \(\Delta _{{\text{r}}} H_{{\text{m}}}^{{{\theta }}} ({\text{s}})\) was calculated as 59.01 ± 0.35 kJ·mol−1 at 298.15 K.
Graphical abstract
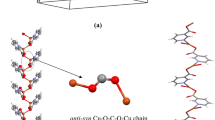
One 1D cobalt (II) coordination polymer was been gained under a simulated condition using an RD496-2000-type microcalorimeter. The synthesis processes of 1 were simulated by calorimetry in situ under four different simulated keeping temperatures to verify the effect of temperature on its formation. The results show that complex 1 has been formed at room temperature, and the hydrothermal processes under different keeping temperatures might be contributed to the formation of its crystals. The solid-state reaction enthalpy change was obtained.








Similar content being viewed by others
References
Han ML, Wen GX, Dong WW, Zhou ZH, Wu YP, Zhao J, Li DS, Ma LF, Bu XH. Heterometallic sodium–europium-cluster-based metal-organic framework as versatile and water stable chemosensor for antibiotics and explosives. J Mater Chem C. 2017;5:8469–74.
Liu XY, Su ZY, Ji WX, Chen SP, Wei Q, Xie G, Yang XW, Gao SL. Structure, physicochemical properties, and density functional theory calculation of high-energy-density materials constructed with intermolecular interaction: nitro group charge determines sensitivity. J Phys Chem C. 2014;41:23487–98.
Ju ZF, Yan SC, Yuan DQ. De novo tailoring pore morphologies and sizes for different substrates in a urea-containing MOFs catalytic platform. Chem Mater. 2016;28:2000–10.
Wu YP, Zhou W, Zhao J, Dong WW, Lan YQ, Li DS, Sun CH, Bu XH. Surfactant-assisted phase- selective synthesis of new cobalt mofs and their efficient electrocatalytic hydrogen evolution reaction. Angew Chem Int Ed. 2017;56:13001–5.
Michaelides A, Skoulika S. Anion-induced formation of lanthanide-organic chains from 3D framework solids. Anion exchange in a crystal-to-crystal manner. Cryst Growth Des. 2009;9:2039–42.
Ren YX, Zhao XL, Wang ZX, Pan Y, Li HP, Wang FY, Zhu SF, Shao CH. Effects of template molecules on the structures and luminescence intensities of a series of porous Tb-MOFs based on 2-nitroterephthalate ligand. RSC Adv. 2018;8:17497–503.
Jiao BJ, Ren YX, Wang WT. Crystal structures and the enthalpy changes of liquid-phase reaction of two energetic zinc (II) complexes based on 5,5′-azoterazolate and two chelated co-ligands. J Mol Struct. 2018;1171:150–4.
Chen SP, He J, Gao SL. Thermochemical study of the solid complexes Ln[(CH3)2NCS2)]3(C12H8N2) (Ln = Eu, Gd, Tb, Dy). J Chem Eng Data. 2008;53:1762–6.
Chen SP, Yang Q, Gao SL. Syntheses, characterization and thermal properties of lanthanide complexes with 2-mercaptonicotinic acid. J Therm Anal Calorim. 2009;95:685–9.
Bhunia MK, Hughes JT, Fettinger JC, Navrotsky A. Thermochemistry of paddle wheel MOFs: Cu-HKUST-1 and Zn-HKUST-1. Langmuir. 2013;29:8140–5.
Chen SP, Ren YX, Wang WT, Gao SL. Nanoporous lanthanide-carboxylate frameworks based on 5-nitroisophthalic acid. Dalton Trans. 2010;39:1552–7.
Gee JA, Sholl DS. Characterization of the thermodynamic stability of solvated metal-organic framework polymorphs using molecular simulations. J Phys Chem C. 2013;117:20636–42.
Fan GC, Jiang JY, Li YF, Huang ZY. Thermodynamic functions of the ZnO nanoweeds. Mater Chem Phys. 2011;130:839–42.
Zhang GH, Wei YG, Wang P, Guo HY. A novel one-dimensional coordination polymer, catena-poly [diaqua-cobalt(II)]-μ-(2,2ʹ-bipyridyl-3,3ʹ-dicarboxylato-κ4N, Nʹ:O, Oʹ). Acta Crystallogr Sect C. 2002;58:m605-7.
Wu BL, Zhang HQ, Zhang HY, Wu QA, Hou HW, Zhu Y, Wang XY. Unique tetradentate coordination of 2,2ʹ-bipyridyl-3,3ʹ-dicarboxylate (bpdc). Hydrothermal synthesis and crystal structure of a novel polymeric supramolecule [Co(bpdc)(H2O)2]n. Aust J Chem. 2003;56:335–8.
Zhang XM, Wu HS, Chen XM. Linear and helical chains in hydrothermally synthesized coordination polymers [Co(bpdc)(H2O)2]n and [Ni(bpdc)(H2O)3]·H2O involving in situ Ligand synthesis. Eur J Inorg Chem. 2003;2003:2959–64.
Siemens S. SAINT: Area Detector control and integration Software. Madison: Siemens Analytical X-ray instruments Inc.; 1996.
Sheldrick GM. SHELXL97 and SHELXTL software reference manual, Version 5.1. Madison: Brucker AXS Inc; 1997.
Sheldrick GM. SADABS. Program for empirical absorption correction of area detector data. Germany: University of Göttingen; 1996.
Ji M, Liu MY, Gao SL, Shi QZ. A new microcalorimeter for measuring thermal effects. Instrum Sci Technol. 2001;29:53–7.
Marthada VK. J Res NBS Standards. 1980;85:467–75.
Gao SL, Chen SP, Hu RZ, Shi QZ. Thermokinetic equation of chemical reaction and its application. Chin J Inorg Chem. 2002;18:362–6.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 6th ed. Hoboken: Wiley; 1991.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21573189) and the National Undergraduate Training Programs of Innovation and Entrepreneurship of Yan’an University (Nos. D2017020 and D2019022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, Y., Yang, X., Wang, Z. et al. Microcalorimetric in situ synthesis and the solid-state reaction enthalpy change for 1D CoII coordination polymer. J Therm Anal Calorim 145, 3293–3299 (2021). https://doi.org/10.1007/s10973-021-10617-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10617-5