Abstract
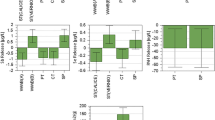
The float–sink density separation method was used to produce a < 1.5 g cm−3 coal density fraction. The sample was subjected to tetralin liquefaction experiments and pyrolysed to produce char samples. The < 1.5 g cm−3 coal density fraction and its liquefaction carbon-rich residue chars formed from the pyrolysis experiments were evaluated using the CO2 gasification tests. The chars were characterized using a thermogravimetric analyzer by heating the samples at isothermal temperatures between 880 and 940 °C under a CO2 atmosphere. The results obtained from the gasification experiments revealed that the gasification reactivity values (Ri, Rs, Rtf, Rt/0.5) of the liquefaction carbon-rich residue chars were higher than those of the < 1.5 g cm−3 coal density fraction chars. Some inherent mineral matter in the residues may play a catalytic role during gasification of South African lighter density waste coal density fractions and their liquefaction residue chars. The initial reactivity of the liquefaction residue chars was observed to be approximately double than those of the < 1.5 g cm−3 coal density fraction chars. Significant increases in a number of pores were associated with the liquefaction experiments of these carbon-rich particles, which aid in the acceleration of the gasification reactions. These increases in the number of pores assisted the reduction of the gasification activation energy values. The RPM was shown to fit the experimental data the best and used to determine kinetic parameters. The gasification activation energy of the < 1.5 g cm−3 coal density fraction chars was shown to be 190.5 kJ mol−1 and 236.7 kJ mol−1 for Highveld float (HF) and Waterberg float (WF) chars, respectively. The values of the apparent activation energy for the liquefaction carbon-rich residues from the same two < 1.5 g cm−3 coal density fraction residue samples were 145.3 kJ mol−1 for the Highveld coal sample and 196.0 kJ mol−1 for the Waterberg coal sample. The gasification results obtained in this study indicate that the possibility of utilizing the waste coal floats and their liquefaction carbon-rich residues in the thermochemical processes (pyrolysis and gasification) is high.








Similar content being viewed by others
References
Khare S, Dell’Amico M. An overview of conversion of residues from coal liquefaction processes. Can J Chem Eng. 2013;91:1660–70.
Uwaoma RC, Strydom CA, Matjie RH, Bunt JR. Influence of density separation of selected South African coal fines on the products obtained during liquefaction using tetralin as a solvent. Energy Fuels. 2019;33:1837–49.
Cui H, Yang J, Liu Z, Bi J. Characteristics of residues from thermal and catalytic coal hydroliquefaction. Fuel. 2003;82:1549–56.
Vasireddy S, Morreale B, Cugini A, Song C, Spivey JJ. Clean liquid fuels from direct coal liquefaction: chemistry, catalysis, technological status, and challenges. Energy Environ Sci. 2011;4:311–45.
Chu XJ, Li W, Li BQ, Chen HK. Gasification property of direct coal liquefaction residue with steam. Process Saf Environ. 2006;84:440–5.
Yan J, Bai Z, Li W, Bai J. Direct liquefaction of a Chinese brown coal and CO2 gasification of the residues. Fuel. 2014;136:280–6.
Itoh H, Hiraide M, Kidoguchi A, Onozaki M, Ishibashi H, Namiki Y, Ikeda K, Inokuchi K, Morooka S. Simulator for coal liquefaction based on the NEDOL process. Ind Eng Chem Res. 2001;40:210–7.
Xu L, Tang M, Liu B, Ma X, Zhang Y, Argyle MD, Fan M. Pyrolysis characteristics and kinetics of residue from China Shenhua industrial direct coal liquefaction plant. Thermochim Acta. 2014;589:1.
Chu X, Li W, Li B, Chen H. Sulfur transfers from pyrolysis and gasification of direct liquefaction residue of Shenhua coal. Fuel. 2008;87:211–5.
Li J, Yang JL, Zhou SF. Li YM Pyrolysis property of solvent extracts from a direct coal liquefaction residue. J Fuel Chem Technol. 2010;38:647–51.
Li XH, Xue YL. LI WY Properties of semi-coke from co-pyrolysis of lignite and direct liquefaction residue of Shendong coal. J Fuel Chem Technol. 2015;43:1281–6.
Zhu P, Luo A, Zhang F, Lei Z, Zhang J, Zhang J. Effects of extractable compounds on the structure and pyrolysis behaviours of two Xinjiang coal. J Anal Appl Pyrolysis. 2018;133:128–35.
Zou L, Jin L, Li Y, Zhu S, Hu H. Effect of tetrahydrofuran extraction on lignite pyrolysis under nitrogen. J Anal Appl Pyrolysis. 2015;112:113–20.
Li J, Yang J, Liu Z. Hydro-treatment of a direct coal liquefaction residue and its components. Catal Today. 2008;130:389–94.
Sugano M, Mashimo K, Wainai T. Upgrading reactions of coal liquefaction residue with basic coal liquid related model compounds. Fuel. 1998;77:447–51.
Xiao N, Zhou Y, Qiu J, Wang Z. Preparation of carbon nanofibers/carbon foam monolithic composite from coal liquefaction residue. Fuel. 2010;89:1169–71.
Xiao N, Zhou Y, Ling Z, Qiu J. Synthesis of a carbon nanofiber/carbon foam composite from coal liquefaction residue for the separation of oil and water. Carbon. 2013;59:530–6.
Ergun S. Kinetics of the reaction of carbon dioxide with carbon. J Phys Chem. 1956;60:480–5.
Koening P, Squires R, Laurendeau N. Char gasification by carbon dioxide. Fuel. 1986;65:412–6.
Radovic L, Jiang H, Lizzio A. A transient kinetics study. References of char gasification in carbon dioxide and oxygen. Energy Fuels. 1991;5:68–74.
Chen S, Yang R, Kapteijn F, Moulijn J. A new surface oxygen complex on carbon: toward a unified mechanism for carbon gasification reactions. Ind Eng Chem. 1993;32:2835–40.
Everson RC, Neomagus HW, Kaitano R, Falcon R, Du Cann VM. Properties of high ash coal-char particles derived from inertinite-rich coal: II. Gasification kinetics with carbon dioxide. Fuel. 2008;87:3403–8.
Everson RC, Okolo GN, Neomagus HW, Dos Santos JM. X-ray diffraction parameters and reaction rate modeling for gasification and combustion of chars derived from inertinite-rich coals. Fuel. 2013;109:148–56.
Uwaoma RC, Strydom CA, Matjie RH, Bunt JR, Okolo GN. Brand DJ pyrolysis of tetralin liquefaction derived residues from lighter density fractions of waste coals taken from waste coal disposal sites in South Africa. Energy Fuels. 2019;33:9074–86.
Roberts MJ, Everson RC, Neomagus HW, Van Niekerk D, Mathews JP, Branken DJ. Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals. Fuel. 2015;142:9–20.
Önal Y, Ceylan K. Effects of treatments on the mineral matter and acidic functional group contents of Turkish lignites. Fuel. 1995;74:972–7.
Wijaya N, Zhang L. A critical review of coal demineralization and its implication on understanding the speciation of organically bound metals and submicrometer mineral grains in coal. Energy Fuels. 2011;25:1–6.
Okolo GN, Everson RC, Neomagus HW, Roberts MJ, Sakurovs R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel. 2015;141:293–304.
Molina A, Mondragón F. Reactivity of coal gasification with steam and CO2. Fuel. 1998;77:1831–9.
Liu G, Benyon P, Benfell KE, Bryant GW, Tate AG, Boyd RK, Harris DJ, Wall TF. The porous structure of bituminous coal chars and its influence on combustion and gasification under chemically controlled conditions. Fuel. 2000;79:617–26.
Meng L, Wang M, Yang H, Ying H, Chang L. Catalytic effect of alkali carbonates on CO2 gasification of Pingshuo coal. Int J Min Sci Technol. 2011;21:587–90.
Cetin E, Moghtaderi B, Gupta R. Wall TF Biomass gasification kinetics: influences of pressure and char structure Combust. Sci Technol. 2005;177:765–91.
Roberts DG, Harris DJ. Char gasification with O2, CO2, and H2O: effects of pressure on intrinsic reaction kinetics. Energy Fuels. 2000;14:483–9.
Levenspiel O. Chemical reaction engineering. 2nd ed. New Delhi: Wiley Eastern Ltd; 1972. p. 357–408.
Wen CY. Noncatalytic heterogeneous solid-fluid reaction models. Ind Eng Chem Res. 1968;60:34–54.
Bhatia SK, Perlmutter DD. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980;26:379–86.
Matjie RH, French D, Ward CR, Pistorius PC, Li Z. Behaviour of coal mineral matter in sintering and slagging of ash during the gasification process. Fuel Process Technol. 2011;92:1426–33.
Spears DA, Duff PD, Caine PM. The West Waterberg tonstein, South Africa. Int J Coal Geo. 1988;9:221–33.
Tsemane MM, Matjie RH, Bunt JR, Neomagus HW, Strydom CA, Waanders FB, Van Alphen C, Uwaoma RC. Mineralogy and petrology of chars produced by South African caking coals and density separated fractions during pyrolysis and their effects on caking propensity. Energy Fuels. 2019;33:7645–58.
Rautenbach R, Strydom CA, Bunt JR, Matjie RH, Campbell QP, Van Alphen C. Mineralogical, chemical, and petrographic properties of selected South African power stations’ feed coals and their corresponding density separated fractions using float-sink and reflux classification methods. Int J Coal Prep Util. 2018. https://doi.org/10.1080/19392699.2018.1533551.
Heller-Kallai L. Reactions of salts with kaolinite at elevated temperatures. I. Clay Miner. 1978;2:221–35.
Zogała A, Janoszek T. CFD simulations of influence of steam in gasification agent on parameters of UCG process. J Sustain Min. 2015;14:2–11.
Uwaoma RC, Strydom CA, Bunt JR, Okolo GN, Matjie RH. The catalytic effect of Benfield waste salt on CO2 gasification of a typical South African Highveld coal. J Therm Anal Calorim. 2018;135:2723–32.
Mangena SJ (2008) Fuel evaluation and coal properties transformation in a Sasol-Lurgi fixed bed dry bottom gasifier operating on North Dakota lignite coal, PhD Thesis. https://repository.nwu.ac.za/bitstream/handle/10394/9720/Mangena_SJ_Chapter_4.pdf?sequence=5&isAllowed=y
Acknowledgements
The authors of this manuscript give thanks to the analysts, researchers and funders following different laboratories and the Department of Science and Technology and National Research Foundation of South Africa: Mrs Belinda Venter of NWU geology laboratories, Bureau Veritas laboratories; SGS laboratories for their support to characterize coal, char and residue samples of this project by X-ray fluorescence and X-ray diffraction equipment; Dr Gregory Okolo for surface area analysis of the solid samples; and DST and NRF for the schemes offering financial assistance to the South African Research Chairs Initiative of the Coal Research Chair Grant No.: 86880 and incentive grant No. 115228. DST and NRF departments are not responsible for the manuscript contents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uwaoma, R.C., Strydom, C.A., Matjie, R.H. et al. Gasification of chars from tetralin liquefaction of < 1.5 g cm−3 carbon-rich residues derived from waste coal fines in South Africa. J Therm Anal Calorim 147, 2353–2367 (2022). https://doi.org/10.1007/s10973-021-10609-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10609-5