Abstract
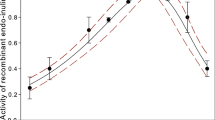
Inulinases catalyze the hydrolysis of inulin to obtain fructose with a yield of about 90–95%. Inulin is a reserve carbohydrate in plant tubers like Jerusalem artichoke, chicory, garlic and onion, leek, rye, barley, dandelion, burdock, banana. The paper presents a comparative analysis to determine the optimum temperatures and the activation energies for various origin exo-inulinases from Aspergillus niger. The parameters were estimated based on the literature of the activity curves versus temperature for hydrolysis of inulin. It was assumed that both the hydrolysis reaction process and the deactivation process of exo-inulinase were first-order reactions by the enzyme concentration. The governing equations are formulated including the activation energy of the deactivation process and temperature effects. A mathematical model describing the effect of temperature on exo-inulinases from Aspergillus niger activity was used. Based on the comparison analysis, values of the activation energies \(E_{\mathrm{a}}\) were in the range from \({25.20 \pm 2.92}\) to \({60.95 \pm 8.30}\,\hbox {kJ}\,\hbox {mol}^{-1}\), the deactivation energies \(E_{\mathrm{d}}\) were in the range from \({80.86 \pm 3.59}\) to \({268.66 \pm 24.06}\,\hbox {kJ}\,\hbox {mol}^{-1}\) and the optimum temperature \(T_{\mathrm{opt}}\) was obtained in the range from \({325.25 \pm 0.41}\) to \({337.35 \pm 0.70\,\hbox {K}}\).







Similar content being viewed by others
References
Ghaderi F, Nemati M, Siahi-Shadbad MR, Valizadeh H, Monajjemzadeh F. Evaluation of activation energy conformity derived from modelfree non-isothermal predictions and Arrhenius isothermal results. J Therm Anal Calorim. 2017;130:1417–27.
S̆imon P, Dubaj T, Cibulková Z. Equivalence of the Arrhenius and non-Arrhenian temperature functions in the temperature range of measurement. J Therm Anal Calorim. 2015;120:231–8.
Hefczyc B, Siudyga T, Zawadiak J, Mianowski A. Analysis of the thermal decomposition of azo-peroxyesters by Arrhenius-type and three-parameter equations. J Therm Anal Calorim. 2011;105:981–6.
Espinosa-Andrews H, Rodríguez-Rodríguez R. Water state diagram and thermal properties of fructans powders. J Therm Anal Calorim. 2018;132:197–204.
Singh P, Gill PK. Production of inulinases: recent advances. Food Technol Biotechnol. 2006;44:151–62.
Chi ZM, Zhang T, Cao TS, Liu XY, Cui W, Zhao CH. Biotechnological potential of inulin for bioprocesses. Bioresour Technol. 2011;102:4295–303.
Rawat HK, Soni H, Treichel H, Kango N. Biotechnological potential of microbial inulinases: recent perspective. Crit Rev Food Sci Nutr. 2017;57:3818–29.
Singh RS, Chauhan K. Production, purification, characterization and applications of fungal inulinases. Curr Biotechnol. 2018;7:242–60.
Chi Z, Chi Z, Zhang T, Liu G, Yue L. Inulinase-expressing microorganisms and applications of inulinases. Appl Microbiol Biotechnol. 2009;82:211–20.
Skowronek M, Fiedurek J. Optimisation of inulinase production by Aspergillus niger using Simplex and classical method. Food Technol Biotechnol. 2004;42:141–6.
Trytek M, Fiedurek J, Podkościelna B, Gawdzik B, Skowronek M. An efficient method for the immobilization of inulinase using new types of polymers containing epoxy groups. J Ind Microbiol Biotechnol. 2015;42:985–96.
Huitrón C, Pérez R, Gutiérrez L, Lappe P, Petrosyan P, Villegas J, Aguilar C, Rocha-Zavaleta L, Blancas A. Bioconversion of Agave tequilana fructans by exo-inulinases from indigenous Aspergillus niger CH-A-2010 enhances ethanol production from raw Agave tequilana juice. J Ind Microbiol Biotechnol. 2013;40:123–32.
Kango N. Production of inulinase using tap roots of dandelion (Taraxacum officinale) by Aspergillus niger. J Food Eng. 2008;85:473–8.
Kumar VV, Premkumar MP, Sathyaselvabala VK, Dineshkirupha S, Nandagopal J, Sivanesan S. Aspergillus niger exo-inulinase purification by three phase partitioning. Eng Life Sci. 2011;11:607–14.
Cattorini S, Marques MPC, Carvalho F, Chheub V, Cabral JMS, Fernandes P. LentikatR-based biocatalysts: effective tools for inulin hydrolysis. Chem Biochem Eng Q. 2009;23:429–34.
Mahmoud DAR, Refaat HW, Abdel-Fattah AF, Mahdy MEE-S, Shousha WG. Novel application of Luffa cylindrica in production of fructose. AJBAS. 2011;5:2127–37.
Arjomand MR, Habibi-Rezaei M, Ahmadian G, Hassanzadeh M, Karkhane AA, Asadifar M, Amanlou M. Deletion of loop fragment adjacent to active site diminishes the stability and activity of exo-inulinase. Int J Biol Macromol. 2016;92:1234–41.
Ricca E, Calabrò V, Curcio S, Iorio G. Optimization of inulin hydrolysis by inulinase accounting for enzyme time- and temperature-dependent deactivation. Biochem Eng J. 2009;48:81–6.
Mazutti M, Ceni G, Di Luccio M, Treichel H. Production of inulinase by solid-state fermentation: effect of process parameters on production and preliminary characterization of enzyme preparations. Bioprocess Biosyst Eng. 2007;30:297–304.
Treichel H, Mazutti MA, Filho FM, Rodrigues MI. Technical viability of the production, partial purification and characterization of inulinase using pretreated agroindustrial residues. Bioprocess Biosyst Eng. 2009;32:425–33.
Wojcik M, Miłek J. A new method to determine optimum temperature and activation energies for enzymatic reactions. Bioprocess Biosyst Eng. 2016;39:1319–23.
Takahashi MB, Leme J, Caricati CP, Tonso A, Núñez EGF, Rocha JC. Artificial neural network associated to UV/Vis spectroscopy for monitoring bioreactions in biopharmaceutical processes. Bioprocess Biosyst Eng. 2015;38:1045–54.
Miłek J. Calculation of temperature optimum as well as activation and deactivation energy for the olive oil hydrolysis with porcine pancreas lipase. Przem Chem. 2020;99:585–7.
Miłek J. Estimation of the kinetic parameters for \(H_{2}O_{2}\) enzymatic decomposition and for catalase deactivation. Braz J Chem Eng. 2018;35:995–1004.
Miłek J. Thermodynamics and kinetics of thermal deactivation of catalase Aspergillus niger. Pol J Chem Technol. 2020;22(2):67–72.
Miłek J. Determination the optimum temperature and activation energy for the hydrolysis of starch catalyzed by \(\alpha \)-amylase Bacillus licheniformis. Przem Chem. 2020;99:880–1.
Miłek J. Determination the optimum temperatures and activation energies of inulin hydrolysis by endo-inulinase Aspergillus niger. Chem Proc Eng. 2020;41:229–36.
Zhou Z, Li A, Bai R, Sun J. Thermal analysis and gelation property of multifunctional epoxy resins used for pultruded composite ropes. J Therm Anal Calorim. 2014;115:1601–8.
Koga N, Goshi Y, Yamada S, Pérez-Maqueda LA. Kinetic approach to partially overlapped thermal decomposition processes. J Therm Anal Calorim. 2013;111:1463–74.
Kayran S, Doymaz I. Determination of drying kinetics and physicochemical characterization of apricot pomace in hot-air dryer. J Therm Anal Calorim. 2017;130:1163–70.
Acknowledgements
The author would like to thank prof. Marek Wójcik for his support and review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miłek, J. Application of the new method to determine the activation energies and optimum temperatures of inulin hydrolysis by exo-inulinases Aspergillus niger. J Therm Anal Calorim 147, 1371–1377 (2022). https://doi.org/10.1007/s10973-020-10495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10495-3