Abstract
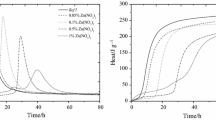
With the increasing use of secondary raw materials and alternative fuels in the cement production industry, the amount of present trash elements rapidly increases. Most of these problematic elements affect the quality of produced clinker or blended cement. This study focuses on zinc and his influence on the hydration process and the formation of new hydration products. Zinc in percentage by mass of OPC 1% and 5% was added in the form of Zn(NO3)2, ZnCl2 and ZnO for modelling of the formation or modification of newly formed products. These new phases are present in mostly trace quantities, and quantification of even well-known phases such as Ca(OH)2 via X-ray diffraction method (XRD) is not suitable due to poor crystallinity and stoichiometry of some zinc compounds related phases. On the other hand, even smaller quantities of known phases could be precisely characterized via simultaneous thermogravimetric (TG) and differential thermal analysis (DTA). Special emphasis was given to mentioned Ca(OH)2 as one of the hydration degree indicators. With a very high content of chloride and nitrate anoint, the hydration, process was not only retarded by zinc but significantly altered causing creation of new phases mainly ettringite analogues with these anoints. In samples with a high dosage of Zn(NO3)2, ZnCl2 even Ca(OH)2 as hydration product cannot be identified by TG–dTG nor XRD. On the other hand with ZnO as zinc source, significant influence on hydration was proven. Initially as retarding agent for early stages of hydration, but also as the agent that increased final degree of hydration relatively to reference OPC.










Similar content being viewed by others
References
Ortego JD, Jackson S, Yu G-S, et al. Solidification of hazardous substances-a TGA and FTIR study of Portland cement containing metal nitrates. J Environ Sci Health Part A Environ Sci Eng. 2008;24:589–602. https://doi.org/10.1080/10934528909375504.
Tashiro C, Oba J, Akama K. The effects of several heavy metal oxides on the formation of ettringite and the microstructure of hardened ettringite. Cem Concrete Res. 1979;9:303–8.
Tashiro C, Oba J. The effects of Cr2O3, Cu(OH)2, ZnO and PbO on the compressive strength and the hydrates of the hardened C3A paste. Cem Concrete Res. 1979;9:253–8.
Gineys N, Aouad G, Damidot D. Managing trace elements in Portland cement—Part II: comparison of two methods to incorporate Zn in a cement. Cem Concrete Compos. 2011;33:629–36.
Bolio-Arceo H, Glasser FP. Zinc oxide in Portland cement. Part II: hydration, strength gain and hydrate mineralogy. Adv Cem Res. 2000;12:173–9. https://doi.org/10.1680/adcr.2000.12.4.173.
Arliguie G, Ollivier JP, Grandet J. Etude de l'effet retardateur du zinc sur l'hydratation de la pate de ciment Portland. Cem Concrete Res. 1982;12:79–86.
Arliguie G, Grandet J. Influence de la composition d'un ciment portland sur son hydration en presence de zinc. Cem Concrete Res. 1990;20:517–24.
Liu J, Jin H, Gu C, Yang Y. Effects of zinc oxide nanoparticles on early-age hydration and the mechanical properties of cement paste. Constr Build Mater. 2019;217:352–62.
Nochaiya T, Sekine Y, Choopun S, Chaipanich A. Microstructure, characterizations, functionality and compressive strength of cement-based materials using zinc oxide nanoparticles as an additive. J Alloys Compd. 2015;630:1–10.
Senff L, Tobaldi DM, Lemes-Rachadel P, Labrincha JA, Hotza D. The influence of TiO2 and ZnO powder mixtures on photocatalytic activity and rheological behavior of cement pastes. Constr Build Mater. 2014;65:191–200.
Rossetti VA, Medici F. Inertization of toxic metals in cement matrices: effects on hydration, setting and hardening. Cem Concrete Res. 1995;25:1147–52.
Trezza MA. Hydration study of ordinary Portland cement in the presence of zinc ions. Mater Res. 2007;10:331–4.
Yousuf M, Mollah A, Pargat JR, Cocke DL. An infrared spectroscopic examination of cement-based solidification/stabilization systems—Portland types V and IP with zinc. J Environ Sci Health Part A Environ Sci Eng Toxicol. 1992;27:1503–19. https://doi.org/10.1080/10934529209375809.
Keppert M, Jerman M, Scheinherrová L, Reiterman P, Doušová B, Černý R. Influence of free and sorbed zinc on cement hydration. J Therm Anal Calorim. 2019;138:1935–43. https://doi.org/10.1007/s10973-019-08200-0.
Ataie FF, Juenger MCG, Taylor-Lange SC, Riding KA. Comparison of the retarding mechanisms of zinc oxide and sucrose on cement hydration and interactions with supplementary cementitious materials. Cem Concrete Res. 2015;72:128–36.
Yousuf M, Mollah A, Vempati RK, Lin T-C, Cocke DL. The interfacial chemistry of solidification/stabilization of metals in cement and pozzolanic material systems. Waste Manag. 1995;15:137–48.
Silatikunsatid T, Jaitanong N, Narksitipan S. A study on influence of zinc oxide in cement composite materials. Key Eng Mater. 2018;772:95–9.
Arliguie G, Grandet J. Etude de l'hydratation du ciment en presence de zinc influence de la teneur en gypse. Cem Concrete Res. 1990;20:346–54.
Nochaiya T, Sekine Y, Choopun S, et al. Microstructure, characterizations, functionality and compressive strength of cement-based materials using zinc oxide nanoparticles as an additive. J Alloys Compd. 2015;630:1–10.
Šiler P, Kolářová I, Másilko J, Novotný R, Opravil T. The effect of zinc on the portland cement hydration. Key Eng Mater. 2018;761:131–4.
Šiler P, Kolářová I, Novotný R, Másilko J, Pořízka J, Bednárek J, et al. Application of isothermal and isoperibolic calorimetry to assess the effect of zinc on cement hydration. J Therm Anal Calorim. 2018;133:27–40. https://doi.org/10.1007/s10973-017-6815-1.
Ouanji F, Khachani M, Arsalane S, Kacimi M, Halim M, El Hamidi A. Synthesis of biodiesel catalyst CaO·ZnO by thermal decomposition of calcium hydroxyzincate dihydrate CaZn2(OH)6·2H2O: kinetic studies and mechanisms. Monatshefte für Chemie Chem Mon. 2016;147:1693–702. https://doi.org/10.1007/s00706-016-1671-4.
Wang S, Yang Z, Zeng L. Study of calcium zincate synthesized by solid-phase synthesis method without strong alkali. Mater Chem Phys. 2008;112:603–6.
Zhu X-M, Yang H-X, Ai X-P, Yu J-X, Cao Y-L. Structural and electrochemical characterization of mechanochemically synthesized calcium zincate as rechargeable anodic materials. J Appl Electrochem. 2003;33:607–12. https://doi.org/10.1023/A:1024999207178.
Lin T-C, Mollah MYA, Vempati RK, et al. Synthesis and characterization of calcium hydroxyzincate using X-ray Diffraction, FT-IR spectroscopy, and scanning force microscopy. Chem Mater. 1995;7:1974–8. https://doi.org/10.1021/cm00058a031.
Birnin-Yauri UA, Glasser FP. Friedel’s salt, Ca2Al(OH)6(Cl, OH)·2H2O: its solid solutions and their role in chloride binding. Cem Concrete Res. 1998;28:1713–23.
López-Salinas E, Serrano MEL, Jácome MAC, Secora IS. Characterization of synthetic hydrocalumite-type [Ca2Al(OH)6]NO3·mH2O: effect of the calcination temperature. J Porous Mater. 1996;2:291–7. https://doi.org/10.1007/BF00489810.
Qoku E, Bier TA, Westphal T. Phase assemblage in ettringite-forming cement pastes: a X-ray diffraction and thermal analysis characterization. J Build Eng. 2017;12:37–50.
Funding
This research has been achieved with the financial support by the Project: GA19-16646S “The elimination of the negative impact of zinc in Portland cement by accelerating concrete admixtures”, with financial support from the Czech science foundation.
Author information
Authors and Affiliations
Contributions
J.Š. were involved in design of an experiment; writing—original draft preparation, discussion of results; P.Š. and I.K. helped with the design of the experiment and supervision in the field of the influence of zinc on the cement matrix; R.N. helped with TG–DTA results in discussion; J.M. was involved in XRD measurement, results and discussion; J.K. contributed to SEM–EDS measurement, results and discussion; J.H contributed to TG–DTA measurements and helped with results discussion; M.J. was involved in hydration stopping and controlled drying procedure of samples before analyses; L.M. helped in sample preparation; and T.O. was involved in methodology and supervision.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Švec, J., Šiler, P., Másilko, J. et al. Simultaneous thermogravimetric and differential thermal analysis determination of products formed during hydration of blended Portland cement doped with zinc. J Therm Anal Calorim 142, 1749–1758 (2020). https://doi.org/10.1007/s10973-020-10253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10253-5