Abstract
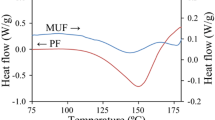
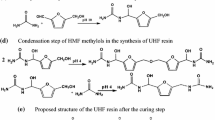
In this work, tea powder was used as a filler to produce low-formaldehyde-emission tea-based melamine-modified urea–formaldehyde resin (TMUF). The thermal stabilities of TMUF and the resin without tea powder (MUF0) were characterized using thermogravimetry. The optimal amount of tea powder m(tea)/m(tea + urea) was equal to 0.15. The formaldehyde emission of the three-layer plywood produced by TMUF was reduced by 75.43%, and the wet bonding strength of the plate was increased by 26.67% as compared with the plywood prepared by MUF0. Compared with MUF0, the thermal degradation peak temperature of TMUF resin was shifted to higher temperature, while the carbon residue was increased. The thermal degradation activation energies of TMUF and MUF0 were similar, while TMUF had a lower pre-exponential A. The Flynn–Wall–Ozawa model-free method is used to estimate various activation energy values at different conversion rates and to predict possible reaction mechanisms. Judging the reaction mechanism according to the slope of the straight line fitted by different models, the best model was based on random nucleation.






Similar content being viewed by others
References
Stefan P, van Hendrikus HWG, Veigel S, Wolfgang GA, Martin R. Urea–formaldehyde microspheres as a potential additive to wood adhesive. J Wood Sci. 2018;64(4):390–7.
Gao Q, Liu C, Luo JL, Li XN, Chen L, Wang W, Li JZ. Effects of resin open time and melamine addition on cold pre-pressing performance of a urea–formaldehyde resin. Eur J Wood Wood Prod. 2018;76(4):1253–61.
Younesi KH, Kazemi NS, Eshkiki RB, Pizzi A. Improving urea formaldehyde resin properties by glyoxalated soda bagasse lignin. Eur J Wood Wood Prod. 2015;73(1):77–85.
Boran S, Usta M, Kaya EG. Decreasing formaldehyde emission from medium density fiberboard panels produced by adding different amine compounds to urea formaldehyde resin. Int J Adhes Adhes. 2011;31(7):674–8.
Ghani A, Ashaari Z, Bawon P, Seng HL. Reducing formaldehyde emission of urea formaldehyde-bonded particleboard by addition of amines as formaldehyde scavenger. Build Environ. 2018;142:188–94.
Hillis WE, Urbach G. The reaction of (+)-catechin with formaldehyde. J Chem Technol Biot. 1959;9(9):474–82.
Takagaki A, Fukai K, Nanjo F, Hara Y. Reactivity of green tea catechins with formaldehyde. J Wood Sci. 2000;46(4):334–8.
Liu L, Jun QI, Zhu Y, Jing Y, Zhang Q, Division P. Optimization of Ultrafine Pulverization Technology in Chaige Tuire Powder. China Pharm. 2017;28(13):1837–41.
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol. 2011;102(10):6230–8.
Huang X, Hoop CFD, Xie J, Hse CY, Feng L. Thermal decomposition characteristics of microwave liquefied rape straw residues using thermogravimetric analysis. J Therm Anal Calorim. 2017;131(2):1911–8.
Findorák R, Fröhlichová M, Legemza J. Thermal degradation and kinetic study of sawdusts and walnut shells via thermal analysis. J Therm Anal Calorim. 2016;125(2):689–94.
Chen YZ, Fan DB, Qin TF, Chu FX. Thermal degradation and stability of accelerated-curing phenol-formaldehyde resin. BioResources. 2014;9:4063–5075.
Gupta GK, Mondal MK. Kinetics and thermodynamic analysis of maize cob pyrolysis for its bioenergy potential using thermogravimetric analyzer. J Therm Anal Calorim. 2019;137(4):1431–41.
Wang K, Deng J, Zhang YN, Wang CP. Kinetics and mechanisms of coal oxidation mass gain phenomenon by TG–FTIR and in situ IR analysis. J Therm Anal Calorim. 2018;132(1):591–8.
Marian E, Tita B, Tita IC, Jurca T, Vicas L. Thermal behaviour and kinetic study of amygdalin. J Therm Anal Calorim. 2018;134(1):765–72.
Ren N, Zhang JJ. Research progress of thermal analysis kinetic data processing methods. Prog Chem. 2006;18(04):410–6.
Yoshida C, Okabe K, Yao T, Shiraishi N, Oya A. Preparation of carbon fibers from biomass-based phenol-formaldehyde resin. J Mater Sci. 2005;40(2):335–9.
Takano T, Murakami T, Kamitakahara H, Nakatsubo F. Mechanism of formaldehyde adsorption of (+)-catechin. J Wood Sci. 2008;54(4):329–31.
Siimer K, Kaljuvee T, Christjanson P, Pehk T. Changes in curing behaviour of aminoresins during storage. J Therm Anal Calorim. 2005;80(1):123–30.
Zorba T, Papadopoulou E, Hatjiissaak A, Paraskevopoulos KM, Chrissafis K. Urea–formaldehyde resins characterized by thermal analysis and FTIR method. J Therm Anal Calorim. 2008;92(1):29–33.
Jiang X, Li C, Chi Y, Yan J. TG–FTIR study on urea–formaldehyde resin residue during pyrolysis and combustion. J Hazard Mater. 2010;173(1–3):205–10.
Chaala A, Yang J, Roy C. Co-pyrolysis of sugarcane bagasse with petroleum residue. Part I: thermogravimetric analysis. Fuel. 2001;80(9):1245–58.
Aouad A, Bilali L, Benchanâa M. Kinetic aspect of thermal decomposition of natural phosphate and its Kerogen. J Therm Anal Calorim. 2002;67(3):733–43.
Yuan JJ, Tu JL, Xu YJ, Qin FGF, Wang CZ. Thermal stability and products chemical analysis of olive leaf extract after enzymolysis based on TG–FTIR and Py-GC–MS. J Therm Anal Calorim. 2018;132(3):1729–40.
Rong L, Peng L, Ho C, Yan SH, Meurens M, Zhang ZZ, Li DX, Wan XC, Bao GH, Gao XL. Brewing and volatiles analysis of three tea beers indicate a potential interaction between tea components and lager yeast. Food Chem. 2016;197:161–7.
Yang HP, Rong Y, Chen HP, Lee DH, Zheng CG. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86(12–13):1781–8.
Carmienke S, Freitag MH, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, Gensichen J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour Technol. 2010;101(12):4584–92.
Wu YZ, Zhao Z, Li HB, He F. Low temperature pyrolysis characteristics of major components of biomass. J Fuel Chem Technol. 2009;37(4):427–32.
Zapata B, Balmaseda J, Fregoso-Israel E, Torres-Garcia E. Thermo-kinetics study of orange peel in air. J Therm Anal Calorim. 2009;98(1):309–15.
Swain SN, Rao KK, Nayak PL. Biodegradable polymers: Part II. Thermal degradation of biodegradable plastics cross-linked from formaldehyde-soy protein concentrate. J Therm Anal Calorim. 2005;79(1):33–8.
Alaba PA, Popoola SI, Abnisal F, Popoola SI, Abnisal F, Lee CS, Ohunakin OS, Adetiba E, Akanle MB, Abdul Patah MF, Atayero AA, Wan D, Wan MA. Thermal decomposition of rice husk: a comprehensive artificial intelligence predictive model. J Therm Anal Calorim. 2020;140(4):1811–23.
Alvarez VA, Vazquez A. Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites. Polym Degrade Stabil. 2004;84(1):13–21.
Ornaghi HL, Ornaghi FG, Neves RM, Monticeli F, Bianchi O. Mechanisms involved in thermal degradation of lignocellulosic fibers: a survey based on chemical composition. Cellulose. 2020;27(9):4949–61.
Zhang JJ, Ren N. A new kinetic method of processing TA data. Chin J Chem. 2004;22(12):1459–62.
Acknowledgements
The authors are very grateful to the financial support from National College Student Innovation Training Program (04070272) and 2019 Research Interests Training (1911113222).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, S., Xiao, H., Chen, Y. et al. Preparation and thermal degradation property analysis of the tea-based melamine-modified urea–formaldehyde (TMUF) resin. J Therm Anal Calorim 146, 1845–1852 (2021). https://doi.org/10.1007/s10973-020-10079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10079-1