Abstract
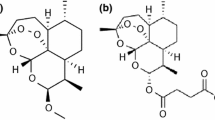
Dihydroartemisinin (DHA), the hydrogenated derivative of the naturally originated sesquiterpene lactone artemisinin, is a well-known antimalarial agent that is currently researched because of its potential as an anticancer medication. Because DHA has been associated with a low oral bioavailability and a short half-life, new formulations meant to overcome these shortcomings are currently under development. As such, the present paper aims to present a comprehensive physico-chemical profile of DHA containing data of great importance for the preformulation stages of the drug design process. As instrumental techniques, attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) spectroscopy and thermal analysis (TG—thermogravimetric/DTG—derivative thermogravimetric/HF—heat flow) were performed, followed by a kinetic study realized using three isoconversional methods (Friedman—Fr, Flynn–Wall–Ozawa—FWO and Kissinger–Akahira–Sunose—KAS), as well as the nonparametric kinetic method (NPK). FTIR spectroscopy confirmed the identity and purity of DHA, and thermal analysis revealed a relatively low thermal stability and a multistep thermooxidation that was proved during the performed kinetic study. The latter unveiled a two-process decomposition determined by both chemical degradations and physical transformations.





Similar content being viewed by others
Abbreviations
- t :
-
Time
- T :
-
Temperature
- α :
-
Conversion degree
- f(α) :
-
The differential conversion function
- R :
-
The universal gas constant
- g(α):
-
The integral conversion function
- β :
-
The heating rate
- k(T):
-
The temperature dependence function
- A :
-
The pre-exponential factor
- E a :
-
The apparent activation energy given by the Arrhenius equation
References
Karunajeewa HA. Artemisinins: artemisinin, dihydroartemisinin, artemether and artesunate. In: Staines HM, Krishna S, editors. Treatment and prevention of Malaria. Basel: Springer; 2012. p. 157–90.
Njuguna NM, Ongarora DSB, Chibale K. Artemisinin derivatives: a patent review (2006–present). Expert Opin Ther Pat. 2012;22(10):1179–203.
Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin—from bench to bedside. Planta Med. 2007;73(4):299–309.
Ho WE, Peh HY, Chan TK, Wong WSF. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142(1):126–39.
Tang W, Eisenbrand G. Chinese drugs of plant origin. chemistry, pharmacology, and use in traditional and modem medicine. 1st ed. Berlin: Springer; 1992.
Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32(13):1655–60.
Cabri W, D’Acquarica I, Simone P, et al. Stereolability of dihydroartemisinin, an antimalarial drug: a comprehensive kinetic investigation. Part 2. J Org Chem. 2011;76(12):4831–40.
Haynes RK. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem. 2006;6(5):509–37.
Jansen FH, Soomro SA. Chemical instability determines the biological action of the artemisinins. Curr Med Chem. 2007;4(30):3243–59.
Dhooghe L, Van Miert S, Jansen H, Vlietinck A, Pieters L. A new decomposition product of dihydroartemisinin. Planta Med. 2007;73(09):10–2.
Wang D, Li H, Gu J, Guo T, Yang S, Guo Z, Zhang X, Zhu W, Zhang J. Ternary system of dihydroartemisinin with hydroxypropyl-β-cyclodextrin and lecithin: simultaneous enhancement of drug solubility and stability in aqueous solutions. J Pharm Biomed Anal. 2013;83:141–8.
Binh TQ, Ilett KF, Batty KT, Davis TME, Hung NC, Powell SM, Thu LTA, Van Thien H, Phuöng HL, Phuong VDB. Oral bioavailability of dihydroartemisinin in Vietnamese volunteers and in patients with falciparum malaria. Br J Clin Pharmacol. 2001;51(6):541–6.
Batty KT, Ilett KF, Davis TME. Protein binding and α:β anomer ratio of dihydroartemisinin in vivo. Br J Clin Pharmacol. 2004;57(4):529–33.
Omwoyo WN, Melariri P, Gathirwa JW, Oloo F, Mahanga GM, Kalombo L, Ogutu B, Swai H. Development, characterization and antimalarial efficacy of dihydroartemisinin loaded solid lipid nanoparticles. Nanomed Nanotechnol Biol Med. 2016;12(3):801–9.
Righeschi C, Coronnello M, Mastrantoni A, Isacchi B, Bergonzi MC, Mini E, Bilia AR. Strategy to provide a useful solution to effective delivery of dihydroartemisinin: development, characterization and in vitro studies of liposomal formulations. Colloids Surf B Biointerfaces. 2014;116:121–7.
Li H, Li X, Shi X, Li Z, Sun Y. Effects of magnetic dihydroartemisinin nano-liposome in inhibiting the proliferation of head and neck squamous cell carcinomas. Phytomedicine. 2019;56(398):215–28.
Wang Z, Duan X, Lv Y, Zhao Y. Low density lipoprotein receptor (LDLR)-targeted lipid nanoparticles for the delivery of sorafenib and Dihydroartemisinin in liver cancers. Life Sci. 2019;239(July):117013.
Ansari MT, Iqbal I, Sunderland VB. Dihydroartemisinin-cyclodextrin complexation: solubility and stability. Arch Pharm Res. 2009;32(1):155–65.
Ansari MT, Imran M, Hassan SSU, Tariq I, Murtaza G. Solubility enhancement of dihydroartemisinin using mixture of hydroxypropyl-β-cyclodextrin and PEG-6000. Lat Am J Pharm. 2014;33(3):483–91.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci Part B Polym Lett. 1969;7(1):41–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett. 1966;4(5):323–8.
Ozawa T. A New Method of Analyzing Thermogravimetric Data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2(3):301–24.
Kissinger HE. Reaction Kinetics in Differential Thermal Analysis. Anal Chem. 1957;29(11):1702–6.
Akahira T, Sunose T. Research report, Trans joint convention of four electrical institutes. Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics model. Thermochim Acta. 1998;316(1):37–45.
Serra R, Sempere J, Nomen R. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal. 1998;52:933–43.
Sempere J, Nomen R, Serra R. Progress in non-parametric kinetics. J Therm Anal Calorim. 1999;56(2):843–9.
Sempere J, Nomen R, Serra R, Soravilla J. The NPK method. Thermochim Acta. 2002;388(1–2):407–14.
Vlase T, Vlase G, Doca N, Bolcu C. Processing of non-isothermal TG data—comparative kinetic analysis with NPK method. J Therm Anal Calorim. 2005;80(1):59–64.
Vlase T, Vlase G, Birta N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88(3):631–5.
Xiao D, Yang B, Zhao YL, Liao XL, Yang XM, Wang F, Chen YJ, Zhou RG. Inclusion complexes of dihydroartemisinin with cyclodextrin and its derivatives: characterization, solubilization and inclusion mode. J Incl Phenom Macrocycl Chem. 2014;79(3–4):349–56.
Circioban D, Ledeti A, Vlase G, Moaca A, Ledeti I, Farcas C, Vlase T, Dehelean C. Thermal degradation, kinetic analysis and evaluation of biological activity on human melanoma for artemisinin. J Therm Anal Calorim. 2018;134(1):741–8.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Ledeti A, Vlase G, Vlase T, Circioban D, Dehelean C, Ledeti I. Kinetic study for solid-state degradation of mental disorder therapeutic agents. J Therm Anal Calorim. 2018;131(1):155–65.
Ledeti I, Ledeti A, Vlase G, Vlase T, Matusz P, Bercean V, Suta LM, Piciu D. Thermal stability of synthetic thyroid hormone l-thyroxine and l-thyroxine sodium salt hydrate both pure and in pharmaceutical formulations. J Pharm Biomed Anal. 2016;125:33–40.
Ledeti I, Vlase G, Vlase T, Bercean V, Fulias A. Kinetic of solid-state degradation of transitional coordinative compounds containing functionalized 1,2,4-triazolic ligand. J Therm Anal Calorim. 2015;121(3):1049–57.
Suta L, Vlase G, Vlase T, et al. Solid State Stability of Cholesterol. Preliminary kinetic analysis. Rev Chim. 2016;67(1):113–5.
Ledeti I, Pusztai AM, Muresan CM, et al. Study of solid-state degradation of prochlorperazine and promethazine. J Therm Anal Calorim. 2018;134(1):731–40.
Cabello CM, Lamore SD, Bair WB, Qiao S, Azimian S, Lesson JL, Wondrak GT. The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest New Drugs. 2012;30(4):1289–301.
Haynes RK, Chan HW, Lung CM, Ng AC, Wong HN, Shek LY, Williams ID, Cartwright A, Gomes MF. Artesunate and dihydroartemisinin (DHA): unusual decomposition products formed under mild conditions and comments on the fitness of DHA as An Antimalarial Drug. ChemMedChem. 2007;2(10):1448–63.
Ledeti A, Olariu T, Caunii A, Vlase G, Circioban D, Baul B, Ledeti I, Vlase T, Murariu M. Evaluation of thermal stability and kinetic of degradation for levodopa in non-isothermal conditions. J Therm Anal Calorim. 2018;131(2):1881–8.
Circioban D, Ledeţi I, Vlase G, Ledeţi A, Axente C, Vlase T, Dehelean C. Kinetics of heterogeneous-induced degradation for artesunate and artemether. J Therm Anal Calorim. 2018;134(1):749–56.
Dickinson CF, Heal GR. A review of the ICTAC kinetics project, 2000. Thermochim Acta. 2009;494(1–2):15–25.
Vyazovkin S. Computational aspects of kinetic analysis. Thermochim Acta. 2000;355(1–2):155–63.
Brown ME, Maciejewski M, Vyazovkin S, et al. Computational aspects of kinetic analysis. Thermochim Acta. 2000;355(1–2):125–43.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3(1):1–12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Circioban, D., Ledeti, A., Vlase, G. et al. Thermal stability and kinetic degradation study for dihydroartemisinin. J Therm Anal Calorim 142, 2131–2139 (2020). https://doi.org/10.1007/s10973-020-09902-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09902-6