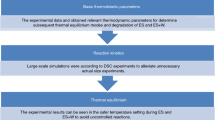
Abstract
In consumer fireworks, various functions of chemicals create an energetic reaction with a type of colored light, smoke, heat, and the sound to achieve the pyrotechnic effects. Fireworks mixtures may be highly sensitive to external disturbances such as ignition sources, temperature changes, shocks, friction, and static electricity. Approximately 95% of fireworks are imported from China by ocean shipment, taking 4 days to a week to Taiwan. The prediction and assessment for the nth-order reaction, and thermokinetic parameters from thermal safety model for fireworks and pyrotechnic compositions were investigated. This study showed which reaction intensity of the propellant as water was added. It can be seen from the results that water will affect the thermal hazard of propellant. Moreover, the novel findings indicated that the water increased the thermal hazard of the propellant and consequently increased the risk for launching a degradation. Based on the heat transfer theoretical models, the time to maximum rate and time to conversion limit were extrapolated for consideration and discussion.










Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor of the Arrhenius equation (s−1)
- C a :
-
Concentration of reactant at time (t\M)
- A(α):
-
Pre-exponential factor at conversion (s−1)
- A′(α):
-
Amended pre-exponential factor by a product of A(α)and f(α) (s−1)
- C p :
-
Specific heat capacity (J g−1 K−1)
- CT:
-
Control temperature (°C)
- E α :
-
Apparent activation energy at specific conversion (kJ mol−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- ET:
-
Emergency temperature (°C)
- f(α):
-
Kinetics function (dimensionless)
- i :
-
Component number (dimensionless)
- k i :
-
Reaction rate constant for ith stage (dimensionless)
- n :
-
Unit outer normal on the boundary (dimensionless)
- n1, n2 :
-
Reaction orders of a specific stage (dimensionless)
- \( Q_{\text{i}}^{\infty } \) :
-
Reaction calorific effect (W)
- q :
-
Rate of heat flow (W)
- q e :
-
Environment (dimensionless)
- R :
-
Gas constant (8.314 J K−1 mol−1)
- r :
-
Reaction rate constant (mol L−1 s−1)
- t :
-
Time (min)
- T :
-
Temperature of sample (K)
- TCL:
-
Time to conversion limit (day)
- T 0 :
-
Apparent exothermic onset temperature (°C)
- T f :
-
Final temperature (K)
- T p :
-
Peak temperature (°C)
- TMR:
-
Time to maximum rate (day, min)
- TMRad :
-
Time to maximum rate under adiabatic conditions (day, min)
- TMRiso :
-
Time to maximum rate under isothermal conditions (day, min)
- U :
-
Heat transfer coefficient (W m−2 K−1)
- W :
-
Heat generation rate (J s−1)
- w :
-
System external (dimensionless)
- x :
-
Unit outer normal on the boundary (dimensionless)
- z :
-
Autocatalytic constant (dimensionless)
- ∆H d :
-
Heat of decomposition (J g−1)
- α :
-
Conversion degree of a component (dimensionless)
- β :
-
Heating rate (°C min−1)
- λ :
-
Thermal conductivity (W m−1 K−1)
- ρ :
-
Density (kg m−3)
References
Billock RM, Chounthirath T, Smith GA. Pediatric firework-related injuries presenting to United States Emergency Departments, 1990–2014. Clin Pediatr. 2017;56(6):535–44.
Conkling JA, Mocella C. Chemistry of pyrotechnics: basic principles and theory. Boca Raton: CRC Press; 1985.
Kumar M, Singh RK, Murari V, Singh AK, Singh RS, Banerjee T. Fireworks induced particle pollution: a spatio-temporal analysis. Atmos Res. 2016;180:78–91. https://doi.org/10.1016/j.atmosres.2016.05.014.
Beveridge A. Forensic investigation of explosions. Boca Raton: CRC Press; 1998.
Sridhar VP, Surianarayanan M, Sivapirakasam SP, Mandal AB. Accelerating rate calorimeter studies of water-induced thermal hazards of fireworks tip mixture. J Therm Anal Calorim. 2013;112(3):1335–41.
Vethathiri Pakkirisamy S, Mahadevan S, Suthangathan Paramashivan S, Asit Baran M. Water induced thermal decomposition of pyrotechnic mixtures–Thermo kinetics and explosion pathway. J Loss Prev Process Ind. 2014;30:275–81. https://doi.org/10.1016/j.jlp.2014.03.005.
Xing Q, Du Z, Zhao L, Yin Q. Study of the environmental humidity influence on the thermal safety of the pyrotechnic compositions. Procedia Eng. 2012;45:552–7. https://doi.org/10.1016/j.proeng.2012.08.202.
Sandvall BK, Jacobson L, Miller EA, Dodge RE, Alex Quistberg D, Rowhani-Rahbar A, et al. Fireworks type, injury pattern, and permanent impairment following severe fireworks-related injuries. Am J Emerg Med. 2017;35(10):1469–73. https://doi.org/10.1016/j.ajem.2017.04.053.
Lin CP, Li J-S, Tseng JM, Mannan MS. Thermal runaway reaction for highly exothermic material in safe storage temperature. J Loss Prev Process Ind. 2016;40:259–65. https://doi.org/10.1016/j.jlp.2016.01.006.
Lin WC, Chen WC, Shu CM. Thermal stability evaluation of multiple tubes of fireworks by calorimetry approaches. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08496-y.
Martín-Alberca C, Zapata F, Carrascosa H, Ortega-Ojeda FE, García-Ruiz C. Study of consumer fireworks post-blast residues by ATR-FTIR. Talanta. 2016;149:257–65. https://doi.org/10.1016/j.talanta.2015.11.070.
Martin-Alberca C, de la Ossa MA, Saiz J, Ferrando JL, Garcia-Ruiz C. Anions in pre- and post-blast consumer fireworks by capillary electrophoresis. Electrophoresis. 2014;35(21–22):3272–80. https://doi.org/10.1002/elps.201400078.
Martín-Alberca C, García-Ruiz C. Analytical techniques for the analysis of consumer fireworks. TrAC Trends Anal Chem. 2014;56:27–36. https://doi.org/10.1016/j.trac.2013.12.010.
Azhagurajan A, Selvakumar N, Thanulingam TL. Thermal and sensitivity analysis of nano aluminium powder for firework application. J Therm Anal Calorim. 2011;105(1):259–67. https://doi.org/10.1007/s10973-011-1435-7.
Liu H, Qian X, Du Z, Huang P, Liu Z. Thermal explosion model and calculation of sphere fireworks and crackers. J Therm Anal Calorim. 2012;110(3):1029–36. https://doi.org/10.1007/s10973-011-2087-3.
Pouretedal HR, Loh Mousavi S. Study of the ratio of fuel to oxidant on the kinetic of ignition reaction of Mg/Ba(NO3)2 and Mg/Sr(NO3)2 pyrotechnics by non-isothermal TG/DSC technique. J Therm Anal Calorim. 2018;132(2):1307–15. https://doi.org/10.1007/s10973-018-7028-y.
Lin W-C, Chen W-C, Shu C-M. Thermal stability evaluation of multiple tubes of fireworks by calorimetry approaches. J Therm Anal Calorim. 2019;138(4):2883–90. https://doi.org/10.1007/s10973-019-08496-y.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77. https://doi.org/10.1016/j.jhazmat.2018.11.003.
Burnham AK. Computational aspects of kinetic analysis: Part D: the ICTAC kinetics project–multi thermal history model fitting methods and their relation to isoconversional methods. Thermochim Acta. 2000;355(1):165–70. https://doi.org/10.1016/S0040-6031(00)00446-9.
Das M, Shu CM. A green approach towards adoption of chemical reaction model on 2, 5-dimethyl-2, 5-di-(tert-butylperoxy) hexane decomposition by differential isoconversional kinetic analysis. J Hazard Mater. 2016;301:222–32.
Fisher H, Forrest H, Grossel SS, Huff J, Muller A, Noronha J, et al. Emergency relief system design using DIERS technology: the design institute for emergency relief systems (DIERS) project manual. Hoboken: Wiley; 2010.
Gao PF, Liu SH, Zhang B, Cao CR, Shu CM. Complex thermal analysis and runaway reaction of 2,2’-azobis (isobutyronitrile) using DSC, STA, VSP2, and GC/MS. J Loss Prev Process Ind. 2019;60:87–95. https://doi.org/10.1016/j.jlp.2019.04.011.
Kossoy A, Belochvostov V, Gustin JL. Methodological aspects of the application of adiabatic calorimetry for thermal safety investigation. J Loss Prev Process Ind. 1994;7(5):397–402. https://doi.org/10.1016/0950-4230(94)80057-X.
Andreozzi R, Caprio V, Somma ID, Sanchirico R. Kinetic and safety assessment for salicylic acid nitration by nitric acid/acetic acid system. J Hazard Mater. 2006;134(1):1–7. https://doi.org/10.1016/j.jhazmat.2005.10.037.
Gonzales NO, Levin ME, Zimmerman LW. The reactivity of sodium borohydride with various species as characterized by adiabatic calorimetry. J Hazard Mater. 2007;142(3):639–46. https://doi.org/10.1016/j.jhazmat.2006.08.058.
Kossoy AA, Koludarova EY. Specific features of kinetics evaluation in calorimetric studies of runaway reactions. J Loss Prev Process Ind. 1995;8(4):229–35. https://doi.org/10.1016/0950-4230(95)00018-V.
Opfermann J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J Therm Anal Calorim. 2000;60(2):641–58.
Martín-Lara M, Blázquez G, Zamora M, Calero M. Kinetic modeling of torrefaction of olive tree pruning. Appl Therm Eng. 2017;113:1410–8.
Chen WC, Shu CM. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J Therm Anal Calorim. 2016;126(3):1937–45.
Kossoy AA, Benin AI, Akhmetshin YG. An advanced approach to reactivity rating. J Hazard Mater. 2005;118(1):9–17. https://doi.org/10.1016/j.jhazmat.2004.08.015.
Andreozzi A, Buonomo B, Manca O. Numerical study of natural convection in vertical channels with adiabatic extensions downstream. Numer Heat Transfer, Part A. 2005;47(8):741–62.
Neylon MK, Savage PE. Analysis of non-isothermal heterogeneous autocatalytic reactions. Chem Eng Sci. 1996;51(6):851–8.
Kossoy AA, Hofelich TC. Methodology and software for assessing reactivity ratings of chemical systems. Process Saf Prog. 2003;22(4):235–40.
Chiang CL, Liu SH, Cao CR, Hou HY, Shu CM. Multiapproach thermodynamic and kinetic characterization of the thermal hazards of 2,2′-azobis(2-methylpropionate) alone and when mixed with several solvents. J Loss Prev Process Ind. 2018;51:150–8. https://doi.org/10.1016/j.jlp.2017.12.003.
Kossoy AA, Akhmetshin YG. Simulation-based approach to design of inherently safer processes. Process Saf Environ Prot. 2012;90(5):349–56. https://doi.org/10.1016/j.psep.2012.03.007.
Kossoy AA, Belokhvostov VM, Koludarova EY. Thermal decomposition of AIBN: Part D: verification of simulation method for SADT determination based on AIBN benchmark. Thermochim Acta. 2015;621:36–43. https://doi.org/10.1016/j.tca.2015.06.008.
Lin CP, Tseng JM. Green technology for improving process manufacturing design and storage management of organic peroxide. Chem Eng J. 2012;180:284–92. https://doi.org/10.1016/j.cej.2011.11.059.
Kossoy AA, Sheinman IY. Effect of temperature gradient in sample cells of adiabatic calorimeters on data interpretation. Thermochim Acta. 2010;500(1):93–9. https://doi.org/10.1016/j.tca.2010.01.003.
Wang TS, Liu SH, Qian XM, You ML, Chou WL, Shu CM. Isothermal hazards evaluation of benzoyl peroxide mixed with benzoic acid via TAM III test. J Therm Anal Calorim. 2013;113(3):1625–31. https://doi.org/10.1007/s10973-013-3020-8.
Huang CC, Peng JJ, Wu SH, Hou HY, You ML, Shu CM. Effects of cumene hydroperoxide on phenol and acetone manufacturing by DSC and VSP2. J Therm Anal Calorim. 2010;102(2):579–85. https://doi.org/10.1007/s10973-010-0953-z.
Duh YS, Kao CS, Lee WLW. Chemical kinetics on thermal decompositions of dicumyl peroxide studied by calorimetry. J Therm Anal Calorim. 2017;127(1):1089–98. https://doi.org/10.1007/s10973-016-5797-8.
Wang SY, Kossoy AA, Yao YD, Chen LP, Chen WH. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim Acta. 2017;655:319–25.
ChemInform Saint-Petersburg (CISP) L. Thermal Safety Software, Available at: http://www.cisp.spb.ru, 2019.
Opfermann J, Hädrich W. Prediction of the thermal response of hazardous materials during storage using an improved technique. Thermochim Acta. 1995;263:29–50.
Acknowledgements
The authors gratefully acknowledge the professional advice received from the members of Process Safety and Disaster Prevention Laboratory (PS&DPL) in Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, CR., Chen, WC. & Shu, CM. Prediction and assessment of fly-up type of fireworks by thermokinetics model. J Therm Anal Calorim 142, 927–936 (2020). https://doi.org/10.1007/s10973-020-09840-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09840-3