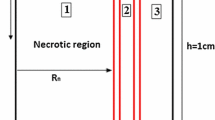
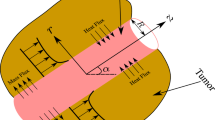
Abstract
High interstitial fluid pressure in the tumor is among the most important barriers to drug delivery. The use of the static magnetic field is one of the treatment modalities for cancers and tumors. In this study, the effect of magnetic field on pressure and magnetized interstitial fluid preservation is discussed. The simulation is solved in two-dimensional space using the COMSOL Multiphysics 5.4.0 software. The geometry includes the porous space of healthy tissue and tumor, as well as the parent vessel as the source and the lymphatic system as the sink modeled based on the biology of the body. Governing coupled equations solved in this simulation include fluid flow in the porous space, which are solved using the law of mass and momentum conservation. The magnetic field equations are solved independently and finally the term of the volume force is coupled to the serial flow equations affecting the interstitial fluid pressure. The concentration distribution is the product of Fick’s equations, which is solved in porous media by the magnetic field according to the bolus injection method. Simulation results show that by applying the static magnetic field generated from permanent magnet to the homogenous magnetic fluid, the magnetized interstitial fluid pressure in the tissue and especially in the tumor region decreases, leading to the higher concentration of the drug that reaches the tumor.

















Similar content being viewed by others
Abbreviations
- \(P_{\text{V}}\) :
-
Intravascular pressure (Pa)
- \(P_{\text{i}}\) :
-
Interstitial pressure (Pa)
- \(P_{\text{L}}\) :
-
Hydrostatic pressure of lymphatic (Pa)
- \(V_{\text{i}}\) :
-
Interstitial fluid velocity (m s−1)
- \(L_{\text{P}}\) :
-
Hydrostatic conductivity of the vessel wall (m Pa−1 s−1)
- \(F\) :
-
Volume force (N)
- \(C\) :
-
Solute Concentration (Kg m−3)
- \(C^{*}\) :
-
Dimensionless concentration
- \(P\) :
-
Diffusive permeability (m s−1)
- \(C_{\text{P}}\) :
-
Plasma concentration of the solute (Kg m−3)
- \(\frac{S}{V}\) :
-
Surface area per unit volume of tissue for transport in the interstitium (1 m−1)
- \(\frac{{L_{\text{PL}} S_{\text{L}} }}{V}\) :
-
The lymphatic filtration coefficient (1 Pa−1 s−1)
- \({\text{Pe}}\) :
-
Peclet number
- \(D_{\text{eff}}\) :
-
Effective diffusion (m2 s−1)
- \(H\) :
-
Magnetic field vector (A m−1)
- \(B\) :
-
Magnetic flux density (T)
- \(J\) :
-
Current density vector (A m−2)
- \(B_{\text{rem}}\) :
-
Remanent magnetic flux density (A m−1)
- \(M\) :
-
Magnetization vector in the blood stream (A m−1)
- \(X\) :
-
Magnetic susceptibility
- \(K\) :
-
Permeability of the porous media (m2)
- \(L_{\text{PL}}\) :
-
Hydrostatic conductivity of the lymphatic vessel wall (m/Pa−1 s−1)
- \(r\) :
-
Length (m)
- \(r^{*}\) :
-
Dimensionless length
- \(v_{\text{vas}}\) :
-
Fluid inlet velocity from blood vascular (m s−1)
- \(v_{\text{L}}\) :
-
Fluid outlet velocity to lymphatic vessel (m s−1)
- \(j_{\text{vas}}\) :
-
Inward flux density of fluid (mol m−2 s−1)
- \(j_{\text{L}}\) :
-
Outward flux density of fluid (mol/m−2 s−1)
- J :
-
Electric current density vector (A m−2)
- i:
-
Interstitial medium
- L:
-
Lymphatic drainage
- vas:
-
Blood vasculature
- eff:
-
Effective
- P:
-
Plasma
- \(\rho\) :
-
Interstitial fluid density (Kg m−3)
- \(\mu\) :
-
Interstitial fluid viscosity (Pa s)
- \(\kappa\) :
-
Hydraulic conductivity of the interstitium (m2 Pa−1 s−1)
- \(\sigma_{\text{f}}\) :
-
Solvent-drag reflection coefficient
- \(\pi_{\text{V}}\) :
-
Osmotic pressure of the plasma (Pa)
- \(\pi_{\text{i}}\) :
-
Osmotic pressure of the interstitial fluid (Pa)
- \(\tau\) :
-
Drug half-life in plasma (h)
- \(\mu_{0}\) :
-
Magnetic permeability of vacuum (N A−2)
- \(\mu_{\text{r}}\) :
-
Relative magnetic permeability of the permanent magnet (N A−2)
- \(\sigma\) :
-
Average osmotic reflection coefficient for the plasma proteins
References
Hadim H, Vafai K. Overview of current computational studies of heat transfer in porous media and their applications–forced convection and multiphase heat transfer. In: Minkowycz WJ, Sparrow EM, editors. Advances in numerical heat transfer, vol 2. Routledge; 2018. p. 291–329.
Vafai K. Handbook of porous media. Boca Raton: CRC Press; 2015.
Vafai K, Tien CL. Boundary and inertia effects on flow and heat transfer in porous media. Int J Heat Mass Transf. 1981;24(2):195–203.
Siavashi M, Joibary SMM. Numerical performance analysis of a counter-flow double-pipe heat exchanger with using nanofluid and both sides partly filled with porous media. J Therm Anal Calorim. 2019;135(2):1595–610.
Khanafer K, Vafai K. Applications of nanofluids in porous medium. J Therm Anal Calorim. 2019;135(2):1479–92.
Rashidi S, Esfahani JA, Rashidi A. A review on the applications of porous materials in solar energy systems. Renew Sustain Energy Rev. 2017;73:1198–210.
Rashidi S, Kashefi MH, Kim KC, Samimi-Abianeh O. Potentials of porous materials for energy management in heat exchangers—a comprehensive review. Appl Energy. 2019;243:206–32.
Sheikholeslami M. New computational approach for exergy and entropy analysis of nanofluid under the impact of Lorentz force through a porous media. Comput Methods Appl Mech Eng. 2019;344:319–33. https://doi.org/10.1016/j.cma.2018.09.044.
Sheikholeslami M, Sheremet MA, Shafee A, Tlili I. Simulation of nanoliquid thermogravitational convection within a porous chamber imposing magnetic and radiation impacts. Physica A. 2020. https://doi.org/10.1016/j.physa.2019.124058.
Vafai K, Tien C. Boundary and inertia effects on convective mass transfer in porous media. Int J Heat Mass Transf. 1982;25(8):1183–90.
Ai L, Vafai K. A coupling model for macromolecule transport in a stenosed arterial wall. Int J Heat Mass Transf. 2006;49(9–10):1568–91.
Yang N, Vafai K. Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int J Heat Mass Transf. 2006;49(5–6):850–67.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Ilami M, Ahmed RJ, Petras A, Beigzadeh B, Marvi H. Magnetic needle Steering in Soft phantom tissue. Sci Rep. 2020;10(1):1–11.
Jang SH, Wientjes MG, Lu D, Au JL-S. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–50.
Blakeslee S. Impenetrable tumors found to block even the newest cancer agents. The New York Times. 1989.
Goldacre R, Sylven B. On the access of blood-borne dyes to various tumour regions. Br J Cancer. 1962;16(2):306.
Sefidgar M, Soltani M, Raahemifar K, Bazmara H, Nayinian SMM, Bazargan M. Effect of tumor shape, size, and tissue transport properties on drug delivery to solid tumors. J Biol Eng. 2014;8(1):12.
Soltani M, Chen P. Numerical modeling of interstitial fluid flow coupled with blood flow through a remodeled solid tumor microvascular network. PLoS ONE. 2013;8(6):e67025.
Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci. 2013;110(46):18632–7.
Jain R. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Controll Release. 2001;74(1–3):7–25.
Pozrikidis C. Numerical simulation of blood and interstitial flow through a solid tumor. J Math Biol. 2010;60(1):75–94.
Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res. 1989;37(1):77–104.
Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res. 1990;40(2):246–63.
Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors: III. Role of binding and metabolism. Microvasc Res. 1991;41(1):5–23.
Saltzman WM, Radomsky ML. Drugs released from polymers: diffusion and elimination in brain tissue. Chem Eng Sci. 1991;46(10):2429–44.
Goh Y-MF, Kong HL, Wang C-H. Simulation of the delivery of doxorubicin to hepatoma. Pharm Res. 2001;18(6):761–70.
Tan WHK, Lee T, Wang CH. Simulation of intratumoral release of etanidazole: effects of the size of surgical opening. J Pharm Sci. 2003;92(4):773–89.
Tan WHK, Wang F, Lee T, Wang CH. Computer simulation of the delivery of etanidazole to brain tumor from PLGA wafers: comparison between linear and double burst release systems. Biotechnol Bioeng. 2003;82(3):278–88.
Teo CS, Tan WHK, Lee T, Wang C-H. Transient interstitial fluid flow in brain tumors: effect on drug delivery. Chem Eng Sci. 2005;60(17):4803–21.
Wang C-H, Li J. Three-dimensional simulation of IgG delivery to tumors. Chem Eng Sci. 1998;53(20):3579–600.
Wang C-H, Li J, Teo CS, Lee T. The delivery of BCNU to brain tumors. J Controll Release. 1999;61(1–2):21–41.
Zhao J, Salmon H, Sarntinoranont M. Effect of heterogeneous vasculature on interstitial transport within a solid tumor. Microvasc Res. 2007;73(3):224–36.
Soltani M, Chen P. Numerical modeling of fluid flow in solid tumors. PLoS ONE. 2011;6(6):e20344.
Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383.
Welter M, Rieger H. Interstitial fluid flow and drug delivery in vascularized tumors: a computational model. PLoS ONE. 2013;8(8):e70395.
Secomb TW, Alberding JP, Hsu R, Dewhirst MW, Pries AR. Angiogenesis: an adaptive dynamic biological patterning problem. PLoS Comput Biol. 2013;9(3):e1002983.
Chaplain MA, McDougall SR, Anderson AR. Blood flow and tumour-induced angiogenesis: dynamically adapting vascular networks. In: Jackson T, editor. Modeling tumor vasculature. New York: Springer; 2012. p. 167–212.
Sefidgar M, Soltani M, Bazmara H, Mousavi M, Bazargan M, Elkamel A. Interstitial flow in cancerous tissue: effect of considering remodeled capillary network. J Tissue Sci Eng Sci. 2014;4:2.
Sefidgar M, Raahemifar K, Bazmara H, Bazargan M, Mousavi S, Soltani M, editors. Effect of remodeled tumor-induced capillary network on interstitial flow in cancerous tissue. In: 2nd Middle East conference on biomedical engineering; 2014: IEEE.
Sefidgar M, Soltani M, Raahemifar K, Sadeghi M, Bazmara H, Bazargan M, et al. Numerical modeling of drug delivery in a dynamic solid tumor microvasculature. Microvasc Res. 2015;99:43–56.
Dejam M. Dispersion in non-Newtonian fluid flows in a conduit with porous walls. Chem Eng Sci. 2018;189:296–310.
Dejam M. Derivation of dispersion coefficient in an electro-osmotic flow of a viscoelastic fluid through a porous-walled microchannel. Chem Eng Sci. 2019;204:298–309.
Forbes ZG, Yellen BB, Halverson DS, Fridman G, Barbee KA, Friedman G. Validation of high gradient magnetic field based drug delivery to magnetizable implants under flow. IEEE Trans Biomed Eng. 2008;55(2):643–9.
Manshadi MK, Saadat M, Mohammadi M, Shamsi M, Dejam M, Kamali R, et al. Delivery of magnetic micro/nanoparticles and magnetic-based drug/cargo into arterial flow for targeted therapy. Drug Deliv. 2018;25(1):1963–73.
Shapiro B. Towards dynamic control of magnetic fields to focus magnetic carriers to targets deep inside the body. J Magn Magn Mater. 2009;321(10):1594–9.
Cherry EM, Eaton JK. A comprehensive model of magnetic particle motion during magnetic drug targeting. Int J Multiph Flow. 2014;59:173–85.
Oldenburg CM, Borglin SE, Moridis GJ. Numerical simulation of ferrofluid flow for subsurface environmental engineering applications. Transp Porous Media. 2000;38(3):319–44.
Morega A, Dobre A, Morega M. Magnetic Field-Flow Interactions in Drug Delivery through an Arterial System. Revue Roumaine des Sciences Techiques—Electrotechnique et Energetique. 2011;56:199–208.
Beigzadeh B, Halabian M. Effect of static magnetic field on the hemodynamic properties of blood flow containing magnetic substances. J Comput Appl Mech. 2016;47(2):181–94.
Shamsi M, Sedaghatkish A, Dejam M, Saghafian M, Mohammadi M, Sanati-Nezhad A. Magnetically assisted intraperitoneal drug delivery for cancer chemotherapy. Drug Deliv. 2018;25(1):846–61.
Shojaee P, Niroomand-Oscuii H, Sefidgar M, Alinezhad L. Effect of nanoparticle size, magnetic intensity, and tumor distance on the distribution of the magnetic nanoparticles in a heterogeneous tumor microenvironment. J Magn Magn Mater. 2020;498:166089.
Beigzadeh B, Rasaeifard A. Homotopy-based solution of Navier–Stokes equations for two-phase flow during magnetic drug targeting. J Mol Liq. 2017;238:11–8.
Pishko GL, Astary GW, Mareci TH, Sarntinoranont M. Sensitivity analysis of an image-based solid tumor computational model with heterogeneous vasculature and porosity. Ann Biomed Eng. 2011;39(9):2360.
Rasouli SS, Jolma IW, Friis HA. Impact of spatially varying hydraulic conductivities on tumor interstitial fluid pressure distribution. Inform Med Unlocked. 2019;16:100175.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosseinikhah, S.M., Beigzadeh, B., Siavashi, M. et al. A numerical study on the effect of static magnetic field on the hemodynamics of magnetic fluid in biological porous media. J Therm Anal Calorim 141, 1543–1558 (2020). https://doi.org/10.1007/s10973-020-09703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09703-x