Abstract
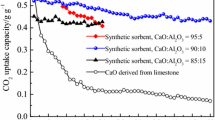
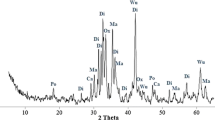
In this work, the SO2 removal performances of the aluminum- and the magnesium-modified carbide slags fabricated by the combustion synthesis from CO2 capture cycles at the calcium looping conditions were investigated in a thermogravimetric analyzer and a dual fixed-bed reactor. The effects of sulfation temperature, number of CO2 capture cycles and calcination condition on the SO2 removal performances of Al- and Mg-modified carbide slags experienced the multiple CO2 capture cycles were studied. The sulfation temperature in the range of 800–950 °C shows a little effect on SO2 removal capacities of Al- and Mg-modified carbide slags experienced the multiple CO2 capture cycles. As the number of CO2 capture cycles increases, the sulfation conversions of the original and modified carbide slags decrease rapidly. However, Al- and Mg-modified carbide slags possess obviously higher SO2 removal capacity and cyclic stability than original carbide slag due to the good supports Ca3Al2O6 and MgO, respectively. And the larger surface areas and volumes of pores in 5–20 nm in diameter of Al- and Mg-modified carbide slags promote the higher SO2 removal capacity than original carbide slag. The modified carbide slag shows higher sintering resistance in high concentration of steam calcination condition during the CO2 capture cycles, compared with high concentration of CO2 calcination condition. Therefore, Al- and Mg-modified carbide slags from CO2 capture cycles based on calcium looping calcined under high steam concentration achieve SO2 removal capacities.








Similar content being viewed by others
References
Li JJ, Zhang YL, Tian YJ, Cheng WJ, Yang JD, Xu DP, Wang YG, Xie KC, Ku AY. Reduction of carbon emissions from China’s coal-fired power industry: insights from the province-level data. J Clean Prod. 2020;242:118518.
Ma ZK, Wu SM, Li YJ. Research progress of CO2 capture with the assist CaO-based energy storage materials at coal-fired power station. Clean Coal Technol. 2019;25(3):1–8.
Blamey J, Anthony EJ, Wang J, Fennell PS. The calcium looping cycle for large-scale CO2 capture. Prog Energy Combust Sci. 2010;36:260–79.
Chen J, Duan LB, Donat F, Muller CR, Anthony EJ, Fan MH. Self-activated, nanostructured composite for improved CaL-CLC technology. Chem Eng J. 2018;351:1038–46.
Chen J, Duan LB, Sun Z. Accurate control of cage-like CaO hollow microspheres for enhanced CO2 capture in calcium looping via a template-assisted synthesis approach. Environ Sci Technol. 2019;53:2249–59.
Przepiórski J, Czyżewski A, Pietrzak R, Tryba B. MgO/CaO-loaded porous carbons for carbon dioxide capture. J Therm Anal Calorim. 2013;111:357–64.
Kaljuvee T, Trikkel A, Kuusik R, Bender V. The role of MgO in the binding of SO2 by lime-containing materials. J Therm Anal Calorim. 2005;80:591–7.
Li YJ, Shi L, Liu CT, He ZR, Wu SM. Studies on CO2 uptake by CaO/Ca3Al2O6 sorbent in calcium looping cycles. J Therm Anal Calorim. 2015;120:1519–28.
Ma XT, Li YJ, Yan XY, Zhang W, Zhao JL, Wang ZY. Preparation of a morph-genetic CaO-based sorbent using paper fibre as a biotemplate for enhanced CO2 capture. Chem Eng J. 2019;361:235–44.
Broda M, Muller CR. Synthesis of highly efficient, Ca-based, Al2O3-stabilized, carbon gel-templated CO2 sorbents. Adv Mater. 2012;24:3059–64.
Pasiut K, Partyka J. The influence of ZrO2 addition on the thermal properties of glass–ceramic materials from SiO2–Al2O3–Na2O–K2O–CaO system. J Therm Anal Calorim. 2017;130:343–50.
Zhang XY, Li ZG, Peng Y, Su WK, Sun XX, Li JH. Investigation on a novel CaO-Y2O3 sorbent for efficient CO2 mitigation. Chem Eng J. 2014;243:297–304.
He DL, Qin CL, Zhang ZH, Pi S, Ran JY, Pu G. Investigation of Y2O3/MxOy-incorporated Ca-based sorbents for efficient and stable CO2 capture at high temperature. Ind Eng Chem Res. 2018;57:11625–35.
Kaygili O, Keser S, Tatar C, Koytepe S, Ates T. Investigation of the structural and thermal properties of Y, Ag and Ce-assisted SiO2-Na2O-CaO-P2O5-based glasses derived by sol–gel method. J Therm Anal Calorim. 2017;128:765–70.
Dweck J, Melchert MBM, Cartledge FK, Leonardo RS, Toledo FRD. A comparative study of hydration kinetics of different cements by thermogravimetry on calcined mass basis. J Therm Anal Calorim. 2017;128:1335–42.
Hu YC, Liu WQ, Chen HQ, Zhou ZJ, Wang WY, Sun J, Yang XW, Li X, Xu MH. Screening of inert solid supports for CaO-based sorbents for high temperature CO2 capture. Fuel. 2016;181:199–206.
Eisinas A, Doneliene J, Baltakys K, Urbutis A. Hydrothermal synthesis of calcium aluminium hydrate-based adsorbent for the removal of CO2. J Therm Anal Calorim. 2018;131:537–44.
Wang NN, Feng YC, Liu L, Guo X. Effects of preparation methods on the structure and property of Al-stabilized CaO-based sorbents for CO2 capture. Fuel Process Technol. 2018;173:276–84.
Kurlov A, Broda M, Hosseini D, Mitchell SJ, Pérez-Ramírez J, Müller CR. Mechanochemically activated, calcium oxide-based, magnesium oxide-stabilized carbon dioxide sorbents. Chemsuschem. 2016;9:2380–90.
Huang L, Zheng QW, Louis B, Wang Q. A facile solvent/nonsolvent preparation of sintering-resistant MgO/CaO composites for high-temperature CO2 capture. Energy Technol. 2018;6:2469–78.
Fernández J, González F, Pesquera C, Neves JA, Viana MM, Dweck J. Qualitative and quantitative characterization of a coal power plant waste by TG/DSC/MS, XRF and XRD. J Therm Anal Calorim. 2016;125:703–10.
Kaljuvee T, Keelman M, Trikkel A, Petkova V. TG-FTIR/MS analysis of thermal and kinetic characteristics of some coal samples. J Therm Anal Calorim. 2013;113:1063–71.
Kaljuvee T, Kuusik R, Trikkel A. SO2 binding into the solid phase during thermooxidation of blendsestonian oil shale semicoke. J Therm Anal Calorim. 2003;72:393–404.
Trikkel A, Keelmann M, Kaljuvee T, Kuusik R. CO2 and SO2 uptake by oil shale ashes. J Therm Anal Calorim. 2010;99:763–9.
Chrissafis K. Multicyclic study on the carbonation of CaO using different limestones. J Therm Anal Calorim. 2007;89:525–9.
Kaljuvee T, Trass O, Pihu T, Konist A, Kuusik R. Activation and reactivity of Estonian oil shale cyclone ash towards SO2 binding. J Therm Anal Calorim. 2015;121:19–28.
Ma SC, Bie X, Sun Y, Gong CQ, Qu BZ. Research progress on flue gas water recovery technology in wet FGD. Clean Coal Technol. 2019;25(1):64–70.
Ryu HJ, Grace JR, Lim CJ. Simultaneous CO2/SO2 capture characteristics of three limestones in a fluidized-bed reactor. Energy Fuels. 2006;20:1621–8.
Li YJ, Wang WJ, Xie X, Sun RY, Wu SM. SO2 retention by highly cycled modified CaO-based sorbent in calcium looping process. J Therm Anal Calorim. 2014;116:955–62.
Ridha FN, Manovic V, Macchi A, Anthony EJ. The effect of SO2 on CO2 capture by CaO-based pellets prepared with a kaolin derived Al(OH)3 binder. Appl Energy. 2012;92:415–20.
Manovic V, Anthony EJ, Loncarevic D. SO2 retention by CaO-based sorbent spent in CO2 looping cycles. Ind Eng Chem Res. 2009;48:6627–32.
Li YJ, Sun RY, Zhao JL, Han KH, Lu CM. Sulfation behavior of white mud from paper manufacture as SO2 sorbent at fluidized bed combustion temperatures. J Therm Anal Calorim. 2012;107:241–8.
Li YJ, Liu CT, Sun RY, Liu HL, Wu SM, Lu CM. Sequential SO2/CO2 capture of calcium-based solid waste from the paper industry in the calcium looping process. Ind Eng Chem Res. 2012;51:16042–8.
Li YJ, Su MY, Xie X, Wu SM, Liu CT. CO2 capture performance of synthetic sorbent prepared from carbide slag and aluminum nitrate hydrate by combustion synthesis. Appl Energy. 2015;145:60–8.
Ma XT, Li YJ, Shi L, He ZR, Wang ZY. Fabrication and CO2 capture performance of magnesia-stabilized carbide slag by by-product of biodiesel during calcium looping process. Appl Energy. 2016;168:85–95.
Wang CB, Zhou X, Jia LF, Tan YW. Sintering of limestone in calcination/carbonation cycles. Ind Eng Chem Res. 2014;53:16235–44.
Sun P, Grace JR, Lim CJ, Anthony EJ. The effect of CaO sintering on cyclic CO2 capture in energy systems. AIChE J. 2007;53:2432–42.
Baker EH. The calcium oxide–carbon dioxide system in the pressure range 1–300 atmospheres. J Chem Soc. 1962;70:464–70.
Wang ZZ, Zhao YY, Sun R, Li YP, Ren XH, Xu J. Effect of reaction conditions on the evolution of surface functional groups in O2/H2O combustion process of demineralized coal char. Fuel Process Technol. 2019;195:106144.
Zhang W, Li YJ, He ZR, Ma XT, Song HP. CO2 capture by carbide slag calcined under high-concentration steam and energy requirement in calcium looping conditions. Appl Energy. 2017;206:869–78.
He DL, Ou ZL, Qin CL, Deng T, Yin JJ, Pu G. Understanding the catalytic acceleration effect of steam on CaCO3 decomposition by density function theory. Chem Eng J. 2020;379:122348.
Donat F, Florin NH, Anthony EJ, Fennell PS. Influence of high-temperature steam on the reactivity of CaO sorbent for CO2 capture. Environ Sci Technol. 2012;46:1262–9.
Champagne S, Lu DY, Symonds RT, Macchi A, Anthony EJ. The effect of steam addition to the calciner in a calcium looping pilot plant. Powder Technol. 2016;290:114–23.
Sun P, Grace JR, Lim CJ, Anthony EJ. Sequential capture of CO2 and SO2 in a pressurized TGA simulating FBC conditions. Environ Sci Technol. 2007;41:2943–9.
Mahuli SK, Agnihotri R, Chauk S, Ghosh-Dastidar A, Wei SH, Fan LS. Pore-structure optimization of calcium carbonate for enhanced sulfation. AIChE J. 1997;43:2323–35.
Acknowledgements
Financial supports from the National Natural Science Foundation of China (51876105) and the Fundamental Research Funds of Shandong University (2018JC039) are gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bian, Z., Li, Y., Wu, S. et al. SO2 removal performances of Al- and Mg-modified carbide slags from CO2 capture cycles at calcium looping conditions. J Therm Anal Calorim 144, 1187–1197 (2021). https://doi.org/10.1007/s10973-020-09622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09622-x