Abstract
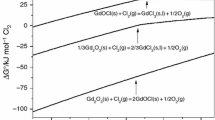
The thermal analysis of the oxide–chloride systems GdCl3–Gd2O3 and GdCl3–KCl–Gd2O3 with the Gd2O3 content up to 0.09 mol fractions was studied in the temperature range 298–1173 K using two methods: differential scanning calorimetry and the method of cooling curves. The liquidus temperatures in these systems have been determined. The solubility of the oxide and the thermodynamic characteristics of the dissolution process were calculated. It was established experimentally that the dissolution of Gd2O3 proceeds by a chemical mechanism, with the formation of GdOCl oxychloride. The received data revealed the main regularities of Gd2O3 solubility in molten GdCl3–KCl–Gd2O3 systems.










Similar content being viewed by others
References
McCarthy GJ, Rhyne JJ, Silber HB. The rare earth in modern science and technology, vol. 2. New York: Springer; 1980. https://doi.org/10.1007/978-1-4613-3054-7.
McCarthy GJ, Rhyne JJ, Silber HB, editors. The rare earth in modern science and technology, vol. 2. New York: Plenum Press; 1980.
Hirota K. Electrochemical deoxidation of RE–O (RE = Gd, Tb, Dy, Er) solid solutions. J Alloy Compd. 1999;282:101–8.
Claux B, Serp J, Fouletier J. Electrochemical reduction of cerium oxide into metal. Electrochim Acta. 2011;56:2771–80.
Wang D. Electrochemical metallization of solid terbium oxide. Angew Chem Int Ed. 2006;45:2384–8.
Baev AE, Novikov GI. Thermodynamic study of rare earth oxychlorides. Zh Neorg Khim. 1969;10(11):2457–64.
Tyen FI, Morozov IS. Interaction of rare earth oxychlorides with their chlorides in the melt. Zh Neorg Khim. 1969;14:2246–52.
Jinqiu YU, Lei CUI, Huaqiang HE, Shihong YAN, Yunsheng HU, Hao WU. Raman spectra of RE2O3 (RE = Eu, Gd, Dy, Ho, Er, Tm, Yb, Lu, Sc and Y): laser-excited luminescence and trace impurity analysis. J Rare Earths. 2014;32:1–4. https://doi.org/10.1016/S1002-0721(14)60025-9.
Korzun IV, Zakir’yanova ID, Nikolaeva EV. Mechanism and caloric effects of the thermal dehydration of GdCl3·6H2O crystalline hydrate. Russ Metall (Metall). 2018;8:722–7. https://doi.org/10.1134/s0036029518080104.
Revzin G.E. Anhydrous chlorides of rare earth elements and scandium. [Bezvodnye hloridy redkozemelnyh elementov i skandia]. In: Methods of obtaining of chemical reagents and preparations [Metody polucheniya himicheskih reaktivov i preparatov]. M.: IREA. 1967. 129 p. [In Rus.].
Barin I, Kubaschewski O. Thermochemical properties of inorganic substances. Berlin: Springer; 1977. https://doi.org/10.1002//bbpc.197800023.
Barin I. Thermochemical data of pure substances. Weinheim: VCH Verlags Gesellschaf; 1989. https://doi.org/10.1002/ange.19901020738.
Seifert HJ. Melting points of lanthanide trichlorides: an unsolved problem. J Therm Anal Calorim. 2005;82:575–80. https://doi.org/10.1007/s10973-005-6946-7.
Rycerz L, Gaune-Escard M. Lanthanide (III) halides: thermodynamic properties and their correlation with crystal structure. J Alloy Compd. 2008;450:167–74. https://doi.org/10.1016/j.jallcom.2006.12.096.
Konings RJM, Kovács A. Thermodynamic properties of the lanthanide (III) halides. Chapter 213. In: Gschneidner KA, Bunzli J-C, Pecharsky V, editors. Handbook on physics and chemistry of rare earths, vol. 33. Amsterdam: Elsevier; 2003.
Brown D. Halides of the lanthanides and actinides. London: Wiley; 1968.
Glushko VP, editor. Thermodynamic properties of individual substances, vol. 4. Moscow: Nauka; 1982.
Ma Zhisen, Sun Yimin, Yan Ding Yu, Wang Zhiyu Qiao, Ye Xinyu. Thermodynamic calculation of the GdCl3–ACl (A = Na, K, Rb, Cs) phase diagrams based on experimental data. Comput Coupling Phase Diagr Thermochem. 2006;30:88–94.
Rycerz L. Practical remarks concerning phase diagrams determination on the basis of differential scanning calorimetry measurements. J Therm Anal Calorim. 2013;113:231–8. https://doi.org/10.1007/s10973-013-3097-0.
I.D. Zakir’yanova, I.V. Korzun. Thermal stability of oxichlorides LnOCl (Ln = Gd, Yb). In: Book of abstracts XXII International conference on chemical thermodynamics in Russia (RCCT 2019) Saint Petersburg, Russia, June 19–23, 2019, p. 287.
Nikolaeva EV, Zakirianova ID, Korzun IV, Bovet AL, Antonov BD. Interaction between barium oxide and barium containing chloride melt. Z Naturforsch. 2015;70(5):325–31. https://doi.org/10.1515/zna-2014-0370.
Nikolaeva EV, Zakiryanova ID, Bovet AL, Korzun IV. On barium oxide solubility in barium-containing chloride melts. Z Naturforsch. 2016;71(8):731–4. https://doi.org/10.1515/zna-2016-0163.
Castrillejo Y, Bermejo MR, Barrado E, Martinez AM, Diaz Arocas P. Solubilization of rare earth oxides in the eutectic LiCl–KCl mixture at 450 C and in the equimolar CaCl2–NaCl melt at 550 C. J Electroanal Chem. 2003;545:141–57. https://doi.org/10.1016/S0022-0728(03)00092-5.
Hase Y, Dunstan P, Temperini M. Raman active normal vibrations of lanthanide oxychlorides. Spectrochim Acta Part A Mol Spectrosc. 1981;37:597–9. https://doi.org/10.1016/0584-8(81)80055-4.
Acknowledgements
The partial financial support of the Russian Foundation for Basic Research, Project № 18-03-00561a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Korzun, I.V., Nikolaeva, E.V. & Zakir’yanova, I.D. Thermal analysis of the oxide–chloride systems GdCl3–Gd2O3 and GdCl3–KCl–Gd2O3. J Therm Anal Calorim 144, 1343–1349 (2021). https://doi.org/10.1007/s10973-020-09558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09558-2