Abstract
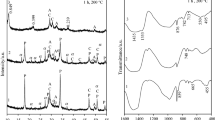
The calcium silicate hydrate gel (C–S–H) was synthesized by the double decomposition method because of the simplicity and the quickness of the procedure. The structure of the C–S–H gels after 1 week and 4 weeks in contact with the formation solution was studied through micro-Raman, Fourier transformed infrared spectroscopy and 29Si nuclear magnetic resonance. Simultaneous thermodifferential–thermogravimetric analysis and mass spectrometry (DTA/TG/MS) was used to identify the amount of calcium carbonate formed due to the reaction between the calcium and atmospheric CO2. With DTA/TG/MS, mass loss due to CO2 was observed to take place at temperatures below 400 °C, unidentified to date, which might be associated with the CO2 adsorbed on the C–S–H gel. Thus, in the TG analysis in the 300–430 °C range, both the loss of water due to the decomposition of the amorphous calcium carbonate and the loss of CO2 adsorbed on the gel must be considered. Additionally, polymerization of the gel and a decrease in the Ca/Si ratio was observed from the samples from 1 to 4 weeks.








Similar content being viewed by others
References
Richardson IG. Model structures for C–(A)–S–H(I). Acta Crystallogr Sect. B. 2014;B70:903–23.
Richardson IG. Tobermorite/jennite- and tobermorite/calcium hydroxide-based models for the structure of C–S–H: applicability to hardened pastes of tricalcium silicate, β-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem Concr Res. 2004;34:1733–77.
Richardson IG. The calcium silicate hydrates. Cem Concr Res. 2008;38:137–58.
Grangeon S, Claret F, Linard Y, Chiaberge C. X-ray diffraction: a powerful tool to probe and understand the structure of nanocrystalline calcium silicate hydrates. Acta Crystallogr Sect. B. 2013;B69:465–73.
Haas J, Nonat A. From C–S–H to C–A–S–H: experimental study and thermodynamic modelling. Cem Concr Res. 2015;68:124–38.
Garrault S, Nonat A. Hydrated layer formation on tricalcium and dicalcium silicate surfaces: experimental study and numerical simulations. Langmuir. 2001;17:8131–8.
Trapote-Barreira A, Cama J, Soler JM. Dissolution kinetics of C–S–H gel: flow-through experiments. Phys Chem Earth. 2014;70–71:17–31.
L’Hôpital E, Lothenbach B, Le Saout G, Kulik D, Scrivener K. Incorporation of aluminium in calcium-silicate-hydrates. Cem Concr Res. 2015;75:91–103.
Komarneni S, Breval E, Roy DM, Roy R. Synthesis and characterization of a 12.6Å calcium silicate hydrate. Cem Concr Res. 1986;16:580–6.
Chen JJ, Thomas JJ, Taylor HFW, Jennings HM. Solubility and structure of calcium silicate hydrate. Cem Concr Res. 2004;34:1499–519.
Sun GK, Young JF, Kirkpatrick RJ. The role of Al in C–S–H: nMR, XRD, and compositional results for precipitated samples. Cem Concr Res. 2006;36:18–29.
Baston GMN, Clacher AP, Heath TG, Hunter FMI, Smith V, Swanton SW. Calcium silicate hydrate (C–S–H) gel dissolution and pH buffering in a cementitious near field. Miner Mag. 2012;76:3045–53.
Hunnicutt W, Struble L, Mondal P. Effect of synthesis procedure on carbonation of calcium-silicate-hydrate. J Am Ceram Soc. 2017;100:3736–45.
Chang J, Fang Y. Quantitative analysis of accelerated carbonation products of the synthetic calcium silicate hydrate (C–S–H) by QXRD and TG/MS. J Therm Anal Calorim. 2015;119:57–62.
Blanco-Varela MT, Aguilera J, Trusilewicz L, Martínez-Ramírez S. Thaumasite formation in hydrated carbonated C3S pastes. Canadá: In XII International Congress of the Chemistry of Cement. Montreal; 2007.
Garbev K, Stemmermann P, Black L, Breen C, Yarwood J, Gasharova B. Structural Features of C–S–H(I) and its carbonation in air-a raman spectroscopic study, Part I: fresh phases. J Am Ceram Soc. 2007;90:900–7.
Black L, Breen C, Yarwood J, Garbev K, Temmermann P, Gasharova B. Structural features of C–S–H(i) and its carbonation in air-a raman spectroscopic study. part ii: carbonated phases. J Am Ceram Soc. 2007;90:908–17.
Yu P, Kirkpatrick RJ, Poe B, McMillan PF, Cong X. Structure of calcium silicate hydrate (C–S–H): near-, mid-, and far-infrared spectroscopy. J Am Ceram Soc. 1999;82:742–8.
Vidmer A, Sclauzero G, Pasquarello A. Infrared spectra of jennite and tobermorite from first-principles. Cem Concr Res. 2014;60:11–23.
Sáez del Bosque IF, Martín-Pastor M, Sobrados I, Martínez-Ramírez S, Blanco-Varela MT. Quantitative analysis of pure triclinic tricalcium silicate and C–S–H gels by 29Si NMR longitudinal relaxation time. Constr Build Mater. 2016;107:52–7.
Martín-Garrido M, Molina-Delgado MT, Martínez-Ramírez S. A comparison between experimental and theoretical Ca/Si ratios in C-S–H and C–S(A)–H gels. J Sol-Gel Sci Tech. 2019. https://doi.org/10.1007/s10971-019-05097-x.
Wehrmeister U, Jacob DE, Soldati AL, Loges N, Häger T, Hofmeister W. Amorphous, nanocrystalline and crystalline calcium carbonates in biological materials. J Raman Spectrosc. 2011;42:926–35.
Martínez-Ramírez S, Sánchez-Cortés S, García-Ramos JV, Domingo C, Fortes C, Blanco-Varela MT. Micro-Raman spectroscopy applied to depth profiles of carbonates formed in lime mortar. Cem Concr Res. 2003;33:2063–8.
García Lodeiro I, Macphee DE, Palomo A, Fernández-Jiménez A. Effect of alkalis on fresh C-S-H gels. FT-IR analysis. Cem Concr Res. 2009;39:147–53.
Monasterio M, Gaitero JJ, Erkizia E, Guerrero Bustos AM, Miccio LA, Dolado JS, Cerveny S. Effect of addition of silica- and amine functionalized silica-nanoparticles on the microstructure of calcium silicate hydrate (C–S–H) gel. J Coll Interface Sci. 2015;450:109–18.
Zhang Z, Xie Y, Xu X, Pan H, Tang R. ransformation of amorphous calcium carbonate into aragonite. J Cryst Growth. 2012;343:62–7.
Morandeau AE, White CE. In situ X-ray pair distribution function analysis of accelerated carbonation of a synthetic calcium-silicate-hydrate gel. J Mater Chem A. 2015;3:8597–605.
Yang X, Cui C, Cui X, Tang G, Ma H. High-temperature phase transition and the activity of tobermorite. J Wuhan Univ Technol-Mat Sci Edit. 2014;29:298–301.
Sabeur H, Saillio M, Vincent J. Thermal stability and microstructural changes in 5 years aged cement paste subjected to high temperature plateaus up to 1000 & #xB0;C as studied by thermal analysis and X-ray diffraction. Heat Mass Transf. 2019;55:2483–501.
Stepkowska ET, Blanes JM, Franco F, Real C, Pérez-Rodríguez JL. Phase transformation on heating of an aged cement paste. Thermochim Acta. 2004;420:79–87.
Roosz C, Gaboreau S, Grangeon S, Prêt D, Montouillout V, Maubec N, Ory S, Blanc P, Vieillard P, Henocq P. Distribution of water in synthetic calcium silicate hydrates. Langmuir. 2016;32:6794–805.
Rodríguez-Navarro C, Elert K, Ševčík R. Amorphous and crystalline calcium carbonate phases during carbonation of nanolimes: implications in heritage conservation. Cryst Eng Comm. 2016;18:6594–607.
Smigelskyte A, Siauciunas R. Parameter influence on the rankinite binder paste and mortar accelerated carbonation curing. J Therm Anal Cal. 2019;138:2651–9.
Dambrauskas T, Baltakys K, Eisinas A. Formation and thermal stability of calcium silicate hydrate substituted with Al3+ ions in the mixtures with CaO/SiO2 = 1.5. J Therm Anal Calorim. 2018;131:501–12.
Pavlík Z, Trník A, Kulovaná T, Scheinherrová L, Rahhal V, Irassar E, Cerný R. DSC and TG Analysis of a blended binder based on waste ceramic powder and portland cement. Int J Thermophys. 2016;37:32–46.
Czoboly O, Lublóy E, Hlavika V, Balázs GL, Kéri O, Szilágyi IM. Fibers and fiber cocktails to improve fire resistance of concrete. J Therm Anal Calorim. 2017;128:1453–61.
Stepkowska ET. Simultaneous IR/TG study of calcium carbonate in two aged cement pastes. J Therm Anal Cal. 2006;84:175–80.
Stepkowska ET. Hypothetical transformation of Ca(OH)2 into CaCO3 in solid-state reactions of Portland Cement. J Therm Anal Cal. 2005;80:727–33.
Sauman Z. Carbonization of porous concrete and its main binding components. Cem Concr Res. 1971;1:645–62.
Thiery M, Villain G, Dangla P, Platret G. Investigation of the carbonation front shape on cementitious materials: effects of the chemical kinetics. Cem Concr Res. 2007;37:1047–58.
Morandeau A, Thiérya M, Dangla P. Investigation of the carbonation mechanism of CH and C–S–H in terms of kinetics, microstructure changes and moisture properties. Cem Concr Res. 2014;56:153–70.
Richardson IG. The nature of C–S–H in hardened cements. Cem Concr Res. 1999;29:1131–47.
Beaudoin JJ, Raki L, Alizadeh RA. 29Si MAS NMR study of modified C–S–H nanostructures. Cem Concr Compos. 2009;31:585–90.
Lippmaa E, Mägi M, Tarmak M, Wieker W, Grimmer ARA. A high resolution 29Si NMR study of the hydration of tricalciumsilicate. Cem Concr Res. 1982;12:597–602.
Acknowledgements
This work was supported by the Comunidad de Madrid and European Social Fund, Program GEOMATERIALES-S-2013/MIT-2914 and MINECO under FIS2017-84318-R. M.M.G. thanks the European Social Fund for funding him.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martín-Garrido, M., Martínez-Ramírez, S. CO2 adsorption on calcium silicate hydrate gel synthesized by double decomposition method. J Therm Anal Calorim 143, 4331–4339 (2021). https://doi.org/10.1007/s10973-020-09374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09374-8