Abstract
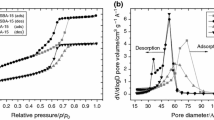
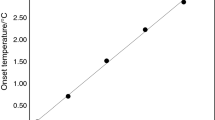
Melting of crystalline compounds inside the nanopores of open-morphology porous systems was studied on a model system, consisted of 1-octadecene and silica gels with different pore sizes, by means of thermogravimetry, differential scanning calorimetry and powder X-ray diffraction. The parameters of silica gels porous structure (surface area, pore size and volume) were calculated using N2 adsorption data. To describe the experimental results, a new thermodynamic model of crystallites melting inside the nanopores of irregular shape was established. This model allows an analytical prediction for the shift of phase transition temperature and melting enthalpy (latent heat of melting) due to the surface tension effects. To a first approximation, both parameters must linearly depend on the specific ratio of the total surface of pores to their total volume, and experimental studies have mostly confirmed this result for the melting of 1-octadecene confined inside the pores of a wide range of various silicas (with the pores of different sizes and geometry).










Similar content being viewed by others
References
Huber P. Soft matter in hard confinement: phase transition thermodynamics, structure, texture, diffusion and flow in nanoporous media. J Phys Condens Matter. 2015;27:103102–43.
Alba-Simionesco C, Coasne B, Dosseh G, Dudziak G, Gubbins KE, Radhakrishnan R, Sliwinska-Bartkowiak MJP. Effects of confinement on freezing and melting. J Phys Condens Matter. 2006;18:15–68.
Hoyt JJ. Effect of stress on melting and freezing in nanopores. Phys Rev Lett. 2006;96:045702–4.
Fakoya MF, Shah SN. Emergence of nanotechnology in the oil and gas industry: emphasis on the application of silica nanoparticles. Petroleum. 2017;3:391–405.
Webber JBW, Dore JC. Neutron diffraction cryoporometry—a measurement technique for studying mesoporous materials and the phases of contained liquids and their crystalline forms. Nucl Instrum Methods Phys Res A. 2008;586:356–66.
Wang D, Sui J, Qi D, Deng S, Wei Y, Wang X, Lan X. Phase transition of docosane in nanopores. J Therm Anal Calorim. 2019;135:2869–77.
Charmas B, Skubiszewska-Zięba J. Application of differential scanning calorimetry to study porous structure of hydrothermally modified silicas. J Therm Anal Calorim. 2017;129:23–32.
Yan X, Wang TB, Pei HR, Wang LP, Lan XZ. Phase behavior of dodecane–tridecane mixtures confined in SBA-15. J Therm Anal Calorim. 2013;113:1297–302.
Zhai M, Zhang S, Sui J, Tian F, Lan XZ. Solid–solid phase transition of tris (hydroxymethyl) aminomethane in nanopores of silica gel and porous glass for thermal energy storage. J Therm Anal Calorim. 2017;129:957–64.
Jiang K, Xie B, Fu D, Luo F, Liu G, Su Y, Wang D. Solid–solid phase transition of n-Alkanes in multiple nanoscale confinement. J Phys Chem B. 2009;114:1388–92.
Alekseev OM, Alekseev SO, Zabashta YF, Lazarenko MM, Hnatiuk KI, Lazarenko MV, Dinzhos RV, Simeonov MS. Influence of open-porous system on the solid-state phase transition in 1-octadecene. Ukr J Phys. 2019;64:340–340.
Jiang Q, Ward MD. Crystallization under nanoscale confinement. Chem Soc Rev. 2014;43:2066–79.
Sґliwinґska-Bartkowiak M, Sterczynґska A, Long Y, Gubbins KE. Influence of microroughness on the wetting properties of nanoporous silica matrices. Mol Phys. 2014;112:2365–71.
Grzabka-Zasadzinґska A, Amietszajew T, Borysiak S. Thermal and mechanical properties of chitosan nanocomposites with cellulose modified in ionic liquids. J Therm Anal Calorim. 2017;130:1–12.
Amanuel S, Bauer H, Bonventre P, Lasher D. Nonfreezing interfacial layers of cyclohexane in nanoporous silica. J Phys Chem C. 2009;113:18983–6.
Krycka KL, Dura JA, Langston LJ, Burba CM. Nanoconfinement-induced phase segregation of binary benzene–cyclohexane solutions within a chemically inert matrix. J Phys Chem C. 2018;122:7676–84.
Mei QS, Lu K. Melting and superheating of crystalline solids: from bulk to nanocrystals. Prog Mater Sci. 2007;52:1175–262.
Thomson W. On the equilibrium of vapour at a curved surface of liquid. Proc R Soc Edinb. 1872;7:63–8.
Jackson CL, McKenna GB. The melting behavior of organic materials confined in porous solids. J Chem Phys. 1990;93:9002–11.
Bulavin LA, Alekseev OM, Zabashta YF, Lazarenko MM. Melting thermodynamics of nanocrystals. J Phys Stud. 2018;22:2601–5.
Balescu R. Equilibrium and nonequilibrium statistical mechanics. Hoboken: Blackwell, Wiley; 1975.
Bulavin LA, Alekseev OM, Zabashta YF, Lazarenko MM. Phase equilibrium, thermodynamic limit, and melting temperature in nanocrystals. Ukr J Phys. 2018;63:1036–1036.
Alekseev OM, Alekseev SO, Bulavin LA, Lazarenko MM, Maiko OM. Phase transitions in chain molecular polycrystals of 1-octadecene. Ukr J Phys. 2008;53:882–7.
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem. 1984;57:603–19.
Pichkur V, Lazarenko M, Alekseev O, Kovbasa V, Lazarenko M. Thermogravimetric research of the extruded and native types of starch. EEJET. 2015;1:52–6.
Lazarenko MM, Alekseev AN, Alekseev SA, Grabovsky YE, Lazarenko MV, Hnatiuk KI. Structure and thermal motion of 1-octadecene, confined in the pores of porous silicon. Mol Cryst Liq Cryst. 2018;674:19–30.
Huber P, Wallacher D, Albers J, Knorr K. Quenching of lamellar ordering in an n-alkane embedded in nanopores. EPL. 2004;65:351–7.
Alekseev AN, Alekseev SA, Zabashta YF, Hnatiuk KI, Dinzhos RV, Lazarenko MM, Grabovskii YE, Bulavin LA. Two-dimensional ordered crystal structure formed by chain molecules in the pores of solid matrix. In: Fesenko O, Yatsenko L, editors. Nanocomposites, nanostructures, and their applications. Springer proceedings in physics, vol. 221. Berlin: Springer; 2018. p. 387–95.
Tkachev SY, Alekseev OM, Lazarenko MM, Lazarenko MV, Kovalov KM, Bokhvan SI, Grabovskii YE, Hoshylyk NV. Topological solitons in branched aliphatic molecules. Mol Cryst Liq Cryst. 2018;665:166–80.
Dinzhos RV, Privalko VP, Privalko EG. Enthalpy relaxation in the cooling/heating cycles of polyamide 6/organoclay nanocomposites. I. Nonisothermal crystallization. J Macromol Sci Phys B. 2005;44:421–30.
Privalko VP, Dinzhos RV, Privalko EG. Enthalpy relaxation in the cooling/heating cycles of polyamide 6/organoclay nanocomposites. II. Melting behavior. J Macromol Sci Phys B. 2005;44:431–43.
Yaws CL. Thermophysical properties of chemicals and hydrocarbons. Chemical, petrochemical & process. Norwich: William Andrew; 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hnatiuk, K.I., Dinzhos, R.V., Simeonov, M.S. et al. Melting of 1-octadecene inside the pores of open-morphology silica gel: thermodynamic model and experimental studies. J Therm Anal Calorim 141, 1243–1250 (2020). https://doi.org/10.1007/s10973-019-09133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09133-4