Abstract
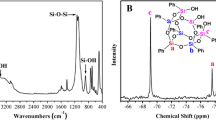
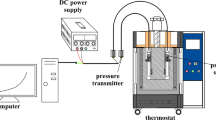
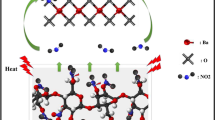
Nitrocellulose (NC), a highly energetic compound, is widely used in nitrolacquer, celluloid, binder, propellants, and explosives. Many studies have focused on the inflammability and instability of NC, but few have investigated its use as a propellant binder. To optimize the properties of NC as a propellant binder, this study introduced 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Bmim][NTf2]) as an additive. This study focuses on the thermal pyrolysis behavior and decomposition mechanism of pure NC (NC-P), NC-NTf2 (i.e., NC mixed with [Bmim][NTf2]), and NC-NTf2-eth (i.e., NC mixed with 30% [Bmim][NTf2] and 70% ethanol). First, the thermal degradation processes of NC and [Bmim][NTf2] were investigated using thermogravimetric analysis. In addition, the essential thermodynamic parameters of NC-P, NC-NTf2, and NC-NTf2-eth were obtained by differential scanning calorimetry, and the apparent activation energies were evaluated using the Starink method and Friedman method. The results show that NC-NTf2 has low onset decomposition temperature, low activation energy, and high energy release rate. Moreover, NC-P, NC-NTf2, and NC-NTf2-eth were characterized by scanning electron microscopy and Fourier transform infrared spectroscopy to verify and interpret the thermal analysis results obtained.













Similar content being viewed by others
Abbreviations
- t :
-
Reaction time (s)
- α :
-
Conversion degree (mass%)
- f(α):
-
Differential form of mechanism function
- G(α):
-
Integral form of mechanism function
- k :
-
Reaction rate constant (s−1)
- A :
-
Pre-exponential factor (s−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- R :
-
Universal gas constant (8.314 J mol K−1)
- β :
-
Heat rate (K min−1)
- T 0 :
-
Onset decomposition temperature (°C)
- T p :
-
Peak decomposition temperature (°C)
- T ed :
-
End decomposition temperature (°C)
- r :
-
Residual mass (mass%)
- Q :
-
Reaction heat release (mJ)
- ∆H :
-
Reaction heat (J g−1)
References
Golubev AE, Kuvshinova SA, Burmistrov VA, et al. Modern advances in the preparation and modification of cellulose nitrates. Russ J Gen Chem. 2018;88:368–81. https://doi.org/10.1134/s1070363218020305.
Wu Y, Luo Y, Ge Z. Properties and application of a novel type of Glycidyl Azide Polymer (GAP)-modified nitrocellulose powders. Propellants Explos Pyrotech. 2015;40:67–73. https://doi.org/10.1002/prep.201400005.
Katoh K, Higashi E, Ariyoshi Y, et al. Relationship between accidents involving spontaneous ignition of nitric acid esters and weather conditions. Sci Technol Energ Mater. 2013;74:132–137.
Dauerman L, Tajima YA. Thermal decomposition and combustion of nitrocellulose. AIAA J. 1968;6:1468–73. https://doi.org/10.2514/3.4790.
Zhang X, Ziemer KS, Weeks BL. Combustion synthesis of N-doped three-dimensional grapheme networks using grapheme networks using grapheme oxide-nitrocellulose composites. Adv Compos Hybrid Mater. 2019;2:492–500. https://doi.org/10.1007/s42114-019-00113-8.
Sovizi MR, Hajimirsadeghi SS, Naderizadeh B. Effect of particle size on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;168:1134–9. https://doi.org/10.1016/j.jhazmat.2009.02.146.
Zhang X, Brandon LW. Preparation of sub-micron nitrocellulose particles for improved combustion behavior. J Hazard Mater. 2014;268:224–8. https://doi.org/10.1016/j.jhazmat.2014.01.019.
Wang K, Liu D, Xu S, et al. Research on the thermal history’s in fluence on the thermal stability of EHN and NC. Thermochim Acta. 2015;610:23–8. https://doi.org/10.1016/j.tca.2015.04.022.
Lin CP, Shu CM. A comparison of thermal decomposition energy and nitrogen content of nitrocellulose in non-fat process of linters by DSC and EA. J Therm Anal Calorim. 2009;95:547–52. https://doi.org/10.1007/s10973-008-9463-7.
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, et al. Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;162:1141–4. https://doi.org/10.1016/j.jhazmat.2008.05.161.
Katoh K, Ito S, Ogata Y, et al. Effect of industrial water components on thermal stability of nitrocellulose. J Therm Anal Calorim. 2010;99:159–64. https://doi.org/10.1007/s10973-009-0492-7.
Katoh K, Soramoto T, Higashi E, et al. Influence of water on the thermal stability of nitrocellulose. Sci Technol Energ Mater. 2014;75(2):44–9.
Nie ZY. Emergency disposal experience and enlightenment of chemical defense in “Tianjin Port 8·12 Explosion Accident”. Chin J Pharmacol Toxicol. 2015. https://doi.org/10.3867/j.issn.1000-3002.2015.05.062.
Zhang H, Duan H, Zuo J, et al. Characterization of post-disaster environmental management for Hazardous Materials Incidents: Lessons learnt from the Tianjin warehouse explosion, China. J Environ Manag. 2017;99:21–30. https://doi.org/10.1016/j.jenvman.2017.05.021.
He Y, He Y, Liu J, et al. Experimental study on the thermal decomposition and combustion characteristics of nitrocellulose with different alcohol humectants. J Hazard Mater. 2017;340:202–12. https://doi.org/10.1016/j.jhazmat.2017.06.029.
Wei RC, He YP, Liu JH, et al. Experimental study on the fire properties of nitrocellulose with different structures. Materials (Basel). 2017;10:316–31. https://doi.org/10.3390/ma10030316.
Wei RC, He YP, Zhang Z, et al. Effect of different humectants on the thermal stability and fire hazard of nitrocellulose. J Therm Anal Calorim. 2018;133:1291–307. https://doi.org/10.1007/s10973-018-7235-6.
Luo QB, Ren T, Shen H, et al. The thermal properties of nitrocellulose: from thermal decomposition to thermal explosion. Combust Sci Technol. 2018;190:579–90. https://doi.org/10.1080/00102202.2017.1396586.
Luo Q, Liang D, Shen H. Evaluation of self-heating and spontaneous combustion risk of biomass and fishmeal with thermal analysis (DSC-TG) and self-heating substances test experiments. Thermochim Acta. 2016;635:1–7. https://doi.org/10.1016/j.tca.2016.04.017.
Guo ML, Ma ZL, He LM, et al. Effect of varied proportion of GAP-ETPE/NC as binder on thermal decomposition behaviors, stability and mechanical properties of nitramine propellants. J Therm Anal Calorim. 2017;130:909–18. https://doi.org/10.1007/s10973-017-6351-z.
Meng XJ, Xiao ZG. Synthesis, thermal properties and sensitivity of ladder-like nitrocellulose grafted by polyethylene glycol. Propellants Explos Pyrotech. 2018;43:300–7. https://doi.org/10.1002/prep.201700193.
Abd-Elghany M, Klapötke TM, Elbeih A. Investigation of 2,2,2-trinitroethyl-nitrocarbamate as a high energy dense oxidizer and its mixture with Nitrocellulose (thermal behavior and decomposition kinetics). J Anal Appl Pyrolysis. 2017;128:397–404. https://doi.org/10.1016/j.jaap.2017.09.010.
Niehaus M. Compounding of glycidyl azide polymer with nitrocellulose and its influence on the properties of propellants. Propellants Explos Pyrotech. 2000;25:236–40. https://doi.org/10.1002/1521-4087(200011)25:5%3c236:AID-PREP236%3e3.0.CO;2-C.
Zhang X, Hikal WM, Zhang Y, et al. Direct laser initiation and improved thermal stability of nitrocellulose/graphene oxide nanocomposites. Appl Phys Lett. 2013;102:141905. https://doi.org/10.1063/1.4801846.
Wang Z, Zhang TF, Zhao BB, et al. Effect of nitrocellulose (NC) on morphology, rheological and mechanical properties of glycidyl azide polymer based energetic thermoplastic elastomer/NC blends. Polym Int. 2017;66:705–11. https://doi.org/10.1002/pi.5312.
Zhang QH, Shreeve JM. Ionic liquid propellants: future fuels for space propulsion. Chem A Eur J. 2013;19:15446–51. https://doi.org/10.1002/chem.201303131.
Matsunaga H, Katoh K, Habu H, et al. Preparation and thermal decomposition behavior of high-energy ionic liquids based on ammonium dinitramide and amine nitrates. Trans Japan Soc Aeronaut Sp Sci Aerosp Technol Jpn. 2018;16:88–92. https://doi.org/10.2322/tastj.16.88.
Duan E, Guo B, Zhang D, et al. Absorption of NO and NO2 in caprolactam tetrabutyl ammonium halide ionic liquids. J Air Waste Manag Assoc. 2011;61:1393–7. https://doi.org/10.1080/10473289.2011.623635.
Zhao GY, Jiang T, Gao HX, et al. Mannich reaction using acidic ionic liquids as catalysts and solvents. Green Chem. 2004;6:75–7.
Vyazovkin S, Burnham AK, Criado JM, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Vyazovkin S, Chrissafis K, Lorenzo MLD, et al. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23. https://doi.org/10.1016/j.tca.2014.05.036.
Lin Y, Liao YF, Yu ZS, et al. Co-pyrolysis kinetics of sewage sludge and oil shale thermal decomposition using TGA-FTIR analysis. Energy Convers Manag. 2016;118:345–52. https://doi.org/10.1016/j.enconman.2016.04.004.
Shen S, Jiang JC, Zhang WX, et al. Process safety evaluation of the synthesis of tert-butyl peracetate. J Loss Prev Process Ind. 2018;54:153–62. https://doi.org/10.1016/j.jlp.2018.03.009.
Huidobro JA, Iglesias I, Alfonso BF, et al. Reducing the effects of noise in the calculation of activation energy by the Friedman method. Chemom Intell Lab Syst. 2016;151:146–52. https://doi.org/10.1016/j.chemolab.2015.12.012.
Stoessel F. Thermal safety of chemical processes: risk assessment and process design. Hoboken: Wiley; 2017.
Ning BK, Liu R, Yang ZQ, et al. Numerical simulation of kinetic parameters of the first-order autocatalytic decomposition of NC. Energ Mater. 1999;7:162–5.
Binke N, Rong L, Zhengquan Y, et al. Studies on the kinetics of the first order autocatalytic decomposition reaction of highly nitrated nitrocellulose. J Therm Anal Calorim. 1999;58:403–11. https://doi.org/10.1023/A:1010163423775.
Lin GB, Zhang CX, Chen LP, et al. Kinetic analysis and self-accelerating decomposition temperature (SADT) of β-nitroso-α-naphthol. Process Saf Environ. 2015;95:69–76. https://doi.org/10.1016/j.psep.2015.02.014.
Acknowledgements
The authors are very grateful for the financial support received from the National Key R&D program, “Study on Safety Control Technology of Storage and Transportation Network of Hazardous Chemicals in Chemical Parks by Multi Species Coupling” (No. 2016YFC0801500), the National Natural Science Foundation of China (21436006, 51834007, 51874181, 51804167), the Jiangsu Natural Science Foundation (BK20150953), the Major Projects of the Natural Science Research for Colleges in Jiangsu Province (17KJA620002), Graduate Student Scientific Research Innovation Project of Jiangsu Province (KYCX19_0829), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, X., Jiang, H., Pan, X. et al. Analysis and characterization of nitrocellulose as binder optimized by 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J Therm Anal Calorim 143, 113–126 (2021). https://doi.org/10.1007/s10973-019-09123-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09123-6