Abstract
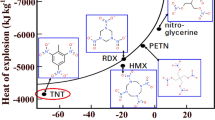
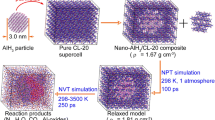
The thermal properties of CL-20 explosive in the bulk and confined in controlled pore glass matrices to nanoscale dimensions were studied using dynamic differential scanning calorimetry. The decomposition reaction of the CL-20 was found to be accelerated in 12-nm-diameter pores compared to the bulk CL-20 with the onset of the decomposition occurring 16–24 °C lower and a fourfold to sevenfold larger reaction rate constant. The total heat of decomposition was found to be independent of pore size and heating rate, and the average activation energy for all samples was found to be 160 ± 7 kJ mol−1.







Similar content being viewed by others
References
Jackson CL, McKenna GB. The melting behavior of organic materials confined in porous solids. J Chem Phys. 1990;93(12):9002–11.
Jackson CL, McKenna GB. The glass transition of organic liquids confined to small pores. J Non-Cryst Solids. 1991;131:221–4.
Di X, Xu B, McKenna GB. The melting behavior of trinitrotoluene nanoconfined in controlled pore glasses. J Therm Anal Calorim. 2013;113(2):533–7.
Xu B, Di X, McKenna GB. Melting of pentaerythritol tetranitrate (PETN) nanoconfined in controlled pore glasses (CPG). J Therm Anal Calorim. 2013;113(2):539–43.
Wang XR, Zhou WS. Glass transition of microtome-sliced thin films. Macromolecules. 2002;35(18):6747–50.
Li QX, Simon SL. Curing of bisphenol M dicyanate ester under nanoscale constraint. Macromolecules. 2008;41(4):1310–7.
Lopez E, Simon Simon SL. Trimerization reaction kinetics and Tg depression of polycyanurate under nanoconfinement. Macromolecules. 2015;48(13):4692–701.
Koh YP, Simon SL. Trimerization of monocyanate ester in nanopores. J Phys Chem B. 2010;114(23):7727–34.
Koh YP, Simon SL. Kinetic study of trimerization of monocyanate ester in nanopores. J Phys Chem B. 2011;115(5):925–32.
Amanuel S, Malhotra VM. Effects of physical confinement (< 125 nm) on the curing behavior of phenolic resin. J Appl Polym Sci. 2006;99(6):3183–6.
Abudakka M, Decker DS, Sutherlin LT, Teeters D. Ceramic/polymer interpenetrating networks exhibiting increased ionic conductivity with temperature control of ion conduction for thermal runaway protection. Int J Hydrogen Energy. 2014;39(6):2988–96.
Cheng SX, McKenna GB. Nano-confinement effects on the glass transition and crystallization behaviors of nifedipine. Mol Pharm. 2019;16(2):856–66.
Bouma RHB, Duvalois W, Van der Heijden AE, Van der Steen AC (2000) Characterization of a commercial grade CL-20: morphology, crystal shape, sensitivity and shock initiation testing by flyer impact. In: 31st international annual conference of ICT’ energetic materials: analysis, diagnostics and testing, Karlsruhe, Germany, 27–30 June 2000, Paper 105-1-105-9. TNO.
Nedelko V, Chukanov N, Raevskii A, Korsounskii B, Larikova T, Kolesova O, Volk F. Comparative investigation of thermal decomposition of various modifications of hexanitrohexaazaisowurtzitane (CL-20). Propellants Explos Pyrotech. 2000;25(5):255–9.
Simpson R, Urtiew P, Ornellas D, Moody G, Scribner K, Hoffman D. CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propellants Explos Pyrotech. 1997;22(5):249–55.
Wardle RB, Hinshaw JC, Braithwaite P, Rose M, Johnston G, Jones R, Poush K. Synthesis of the caged nitramine HNIW (CL-20). In: 27th international annual conference on ICT (proceedings), Jahrestagung, Karlsruhe 1996 Jun 25, vol 27, pp 1–10.
Patil DG, Brill TB. Thermal decomposition of energetic materials 53. Kinetics and mechanism of thermolysis of hexanitrohexazaisowurtzitane. Combust Flame. 1991;87(2):145–51.
Rice JK, Russell T. High-pressure matrix isolation of heterogeneous condensed phase chemical reactions under extreme conditions. Chem Phys Lett. 1995;234(1–3):195–202.
Turcotte R, Vachon M, Kwok QS, Wang R, Jones DE. Thermal study of HNIW (CL-20). Thermochim Acta. 2005;433(1–2):105–15.
Ordzhonikidze O, Pivkina A, Frolov Y, Muravyev N, Monogarov K. Comparative study of HMX and CL-20. J Therm Anal Calorim. 2011;105(2):529–34.
Gao B, Wang DJ, Zhang J, Hu YJ, Shen JP, Wang J, Huang B, Qiao ZQ, Huang H, Nie FD, Yang GC. Facile, continuous and large-scale synthesis of CL-20/HMX nano co-crystals with high-performance by ultrasonic spray-assisted electrostatic adsorption method. J Mater Chem A. 2014;2(47):19969–74.
Lobbecke S. Thermal behavior and stability of HNIW (CL-20). In: 29th international annual conference of ICT, 1998, Karlsruhe, 1998, vol. 145, pp. 1–15.
Lee JS, Hsu CK, Chang CL. A study on the thermal decomposition behaviors of PETN, RDX, HNS and HMX. Thermochim Acta. 2002;392:173–6.
Oxley JC, Kooh AB, Szekeres R, Zheng W. Mechanisms of nitramine thermolysis. J Phys Chem. 1994;98:7004–8.
Geetha M, Nair UR, Sarwade DB, Gore GM, Asthana SN, Singh HJ. Studies on CL-20: the most powerful high energy material. J Therm Anal Calorim. 2003;73(3):913–22.
Foltz MF, Coon CL, Garcia F, Nichols AL III. The thermal stability of the polymorphs of exanitrohexaazaisowurtzitane, Part II. Propellants Explos Pyrotech. 1994;19(3):133–44.
Burnham AK, Weese RK. Thermal decomposition kinetics of HMX. Lawrence Livermore National Lab (LLNL), Livermore, CA (United States); 2004 Nov 18.
Bhattacharia SK, Weeks BL, Chen CC. Melting behavior and heat of fusion of compounds that undergo simultaneous melting and decomposition: an investigation with HMX. J Chem Eng Data. 2017;62(3):967–72.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Hamilton BD, Ha JM, Hillmyer MA, Ward MD. Manipulating crystal growth and polymorphism by confinement in nanoscale crystallization chambers. Acc Chem Res. 2011;45(3):414–23.
Hamilton BD, Hillmyer MA, Ward MD. Glycine polymorphism in nanoscale crystallization chambers. Cryst Growth Des. 2008;8(9):3368–75.
Ha JM, Wolf JH, Hillmyer MA, Ward MD. Polymorph selectivity under nanoscopic confinement. J Am Chem Soc. 2004;126(11):3382–3.
Pallaka MR, Unruh DK, Simon SL. Melting behavior of n-alkanes in anodic aluminum oxide (AAO) nanopores using Flash differential scanning calorimetry. Thermochim Acta. 2018;10(663):157–64.
Acknowledgements
The authors gratefully acknowledge Dr. Victor Stepanov from Picatinny Arsenal for supplying the CL-20 material and for helpful suggestions in the preparation of the manuscript. We also thank Dr. Yung P. Koh at Texas Tech University for his help with the calorimetry and Dr. Kaz Surowiec for performing HPLC to test the purity of CL-20, in the Department of Chemistry and Biochemistry at Texas Tech University. We also thank the National Armaments Consortium (NAC) and the Defense Ordnance Technology Consortium (DOTC) for funding under agreement DOTC-16-01-INIT0177. Also, at Texas Tech University, the John R. Bradford Endowment, the Whitacre College of Engineering, the Department of Chemical Engineering, and the Office of Research and Innovation are thanked for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bari, R., Denton, A.A., Fondren, Z.T. et al. Acceleration of decomposition of CL-20 explosive under nanoconfinement. J Therm Anal Calorim 140, 2649–2655 (2020). https://doi.org/10.1007/s10973-019-09027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09027-5