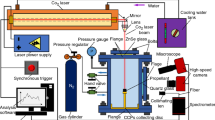
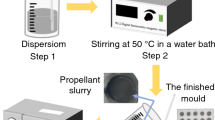
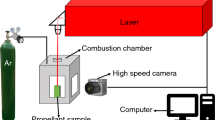
Abstract
Differences of thermal decomposition characteristics and combustion properties between CL-20-based propellants and HMX-based propellants were researched by combination of theory and practice. Burning rates and burning rate pressure exponents of CL-20-based propellants were much higher than those of HMX-based propellants, when contents and particle sizes of CL-20 and HMX were identical. The thermal decomposition of CL-20 and CL-20-based propellants was systematically studied by comparison of HMX and HMX-based propellants. HMX melted firstly at 198.53 °C and then decomposed violently at 284.3 °C, while CL-20 only decomposed violently at 246.9 °C without any melting peak, and the heat released from decomposition of CL-20 was much higher than that of HMX. Thermal decomposition of CL-20 or HMX could be greatly enhanced by GAP. CL-20 could accelerate the high-temperature decomposition of AP, whereas the decomposition of HMX was greatly enhanced by AP. Mole ratio of [NO2]/[N2O] in decomposition gas products of CL-20 was 3.07, whereas the result for HMX was 0.43. More oxidative gases were generated for CL-20 or CL-20-based propellants. Molar reaction heat in luminescent flame zone and dark zone for CL-20-based propellants was much higher than that for HMX-based propellants, resulting in a higher burning rate for CL-20-based propellants. Value of [NO2]/[N2O] for gas-phase products of thermal decomposition of CL-20/ammonium perchlorate (AP) mixed system was also much higher than of HMX/AP mixed system, resulting in more oxidative gases, such as NO2, involved in the thermal decomposition and combustion of CL-20-based propellants with ammonium perchlorate, further leading to higher burning rates of CL-20-based propellants.














Similar content being viewed by others
References
Nair UR, Sivabalan R, Gore GM, Geetha M, Asthana SN, Singh H. Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-based formulations (review). Combust Explos Shock Waves. 2005;41:121–32.
Geetha M, Nair UR, Sarwade DB, Gore GM, Asthana SN. Studies on CL-20: The most powerful high energy material. J Therm Anal Calorim. 2003;73:913–22.
He X, Liu Y, Huang SL, Liu Y, Pu XM, Xu T. Raman spectroscopy coupled with principal component analysis to quantitatively analyze four crystallographic phases of explosive CL-20. RSC Adv. 2018;8:23348–52.
Trache M, Klapötke TM, Maiz L, AbdElghany M, DeLuca LT. Recent advances in new oxidizers for solid rocket propulsion. Green Chem. 2017;19:4711–36.
Lu YY, Shu YJ, Liu N, Lu XM, Xu MH. Molecular dynamics simulations on ε-CL-20-based PBXs with added GAP and its derivative polymers. RSC Adv. 2018;8:4955–62.
Hussein AK, Elbeih A, Zeman S. The effect of glycidyl azide polymer on the stability and explosive properties of different interesting nitramines. RSC Adv. 2018;8:17272–8.
Chen T, Zhang Y, Guo SF, Zhao LM, Chen W, Hao GZ, Xiao L, Ke X, Jiang W. Preparation and property of CL-20/BAMO-THF energetic nanocomposites. Def Technol. 2019;15:306–12.
Tomoki N, Makoto K. Influences of particle size and content of HMX on burning characteristics of HMX-based propellant. Aerosp Sci Technol. 2013;27:209–15.
Chatterjee S, Deb U, Datta S, Walther C, Gupta DK. Common explosives (TNT, RDX, HMX) and their fate in the environment: emphasizing bioremediation. Chemosphere. 2017;184:428–51.
Turcotte R, Vachon M, Kwok QSM. Thermal study of HNIW (CL-20). Thermochim Acta. 2005;433:105–15.
Naik NH, Gore GM, Gandhe BR, Sikder AK. Studies on thermal decomposition mechanism of CL-20 by pyrolysis gas chromatography–mass spectrometry (Py-GC/MS). J Hazard Mater. 2008;159:630–5.
Yang R, Xiao H. Dissociation mechanism of HNIW ions investigated by chemical ionization and electron impact mass spectroscopy. Propellants Explos Pyrotech. 2010;31:148–54.
Chen SL, Liu JB, Wei SQ, Qiang SU. Study on thermal decomposition kinetics of hexanitrohexaazaisowurtzitane. Chin J Energ Mater. 2002;10:46–8.
Washburn EB, Parr TP, HansonParr DM. Micro videographic analysis of the melt layer of self-deflagrating HMX and RDX. Propellants Explos Pyrotech. 2010;35:46–52.
Kalman J, Essel J. Influence of particle size on the combustion of CL-20/HTPB propellants. Propellants Explos Pyrotech. 2017;42:1261–7.
Nedelko VV, Chukanov NV, Raevskii AV, Korsounskii BL, Larikova TS, Kolesova OI. Comparative investigation of thermal decomposition of various modifications of hexanitrohexaazaisowurtzitane (CL-20). Propellants Explos Pyrotech. 2015;25:255–9.
Kuo KK, Acharya R. Applications of turbulent and multiphase combustion. Hoboken: Wiley; 2012.
Korobeinichev OP, Paletskii AA, Volkov EN. Flame structure and combustion chemistry of energetic materials. Russ J Phys Chem B. 2008;2:206–28.
Homan BE, Miller MS, Vanderhoff JA. Flame absorption diagnostics and modeling investigations of RDX flame structure. Combust Flame. 2000;120:301–17.
Paletsky AA, Tereshchenko AG, Volkov EN, Korobeinichev OP, Sakovich GV, Komarov VF, et al. Study of the CL-20 flame structure using probing molecular beam mass spectrometry. Combust Explos Shock Waves. 2009;45:286–92.
Zhang T, Li YY, Wang W, Guo Y, Li CC, Pang AM, Guo ZQ, Ma HX. Directional assembly of flowerlike maghemite on graphene and its catalytic activity for the thermal decomposition of CL-20. Ceram Int. 2019;45:20606–12.
Ding L, Zhao FQ, Pan Q, Xu HX. Research on the thermal decomposition behavior of NEPE propellant containing CL-20. J Anal Appl Pyrolysis. 2016;121:121–7.
Gump JC, Peiris SM. Phase transitions and isothermal equations of state of epsilon hexanitrohexaazaisowurtzitane (CL-20). J Appl Phys. 2008;104:083509.
Zhang T, Guo Y, Li J, Guan Y, Guo Z, Ma H. High catalytic activity of nitrogen-doped graphene on the thermal decomposition of CL-20. Propellants Explos Pyrotech. 2018;43:1263–9.
Mei X, Jiao Q, Zhu Y, Yu J, Chen L. Calorimetry thermal study of HNIW(CL-20) and mixtures containing aluminum powder. J Therm Anal Calorim. 2014;116:1159–63.
Xing XL, Zhao FQ, Ma SN, et al. Thermal decomposition behavior, kinetics, and thermal hazard evaluation of CMDB propellant containing CL-20 by microcalorimetry. J Therm Anal Calorim. 2012;110:1451–5.
Stewart JJP. Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J Mol Model. 2013;19:1–32.
Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor Chem Acc. 2008;119:525.
Hegab A, Jackson TL, Buckmaster J, Stewart DS. Nonsteady burning of periodic sandwich propellants with complete coupling between the solid and gas phases. Combust Flame. 2001;125:1055–70.
Kim JH, Yim YJ. Effect of particle size on the thermal decomposition of EPSILON-hexanitrohexaazaisowurtzitane. J Chem Eng Japan. 1999;32:237–41.
Acknowledgements
The work was supported by the Natural Science Foundation of China (Grant No. 51701067).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10973_2019_9004_MOESM1_ESM.pdf
Theoretical calculation of thermal decomposition behaviors of HMX and CL-20 was demonstrated in Supporting Information. (PDF 440 kb)
Rights and permissions
About this article
Cite this article
Zhou, S., Zhou, X., Tang, G. et al. Differences of thermal decomposition behaviors and combustion properties between CL-20-based propellants and HMX-based solid propellants. J Therm Anal Calorim 140, 2529–2540 (2020). https://doi.org/10.1007/s10973-019-09004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09004-y