Abstract
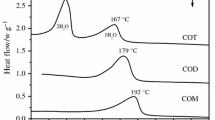
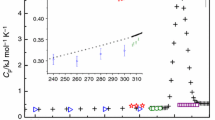
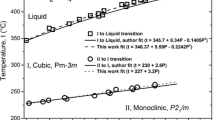
Calorimetric measurements of sodium chloride dihydrate NaCl·2H2O (mineral name hydrohalite) were carried out with using DSC. Heat capacity from 190 to 250 K was measured and found to increase from 109 to 137 J mol−1 K−1. The enthalpy of formation of hydrohalite from solid ice and halite at 273.15 K was derived from the thermal effect of melting/decomposition in DSC measurements and found to be close to − 1.8 kJ mol−1. The same DSC results show clearly that the upper temperature limit for the existence of hydrohalite is several degrees greater than the current value of 273.15 K accepted for the peritectic decomposition of hydrohalite. The phase diagram of the NaCl–H2O system needs correction.


Similar content being viewed by others
References
Carns RC, Light B, Warren SG. The spectral albedo of sea ice and salt crusts on the tropical ocean of Snowball Earth: II. Optical modeling. J Geophys Res Oceans. 2016;121:5217–30. https://doi.org/10.1002/2016JC011804.
Shields AL, Carns RC. Hydrohalite salt-albedo feedback could cool M-dwarf planets. Astrophys J. 2018;867:11. https://doi.org/10.3847/1538-4357/aadcaa.
Valenti P, Bodnar RJ, Schmidt C. Experimental determination of H2O–NaCl liquidi to 25 mass% NaCl and 1.4 GPa: application to the Jovian satellite Europa. Geochim Cosmochim Acta. 2012;92:117–28. https://doi.org/10.1016/j.gca.2012.06.007.
Flahaut J, Martinot M, Bishop JL, Davies GR, Potts NJ. Remote sensing and in situ mineralogic survey of the Chilean salars: an analog to Mars evaporate deposits? Icarus. 2017;282:152–73. https://doi.org/10.1016/j.icarus.2016.09.041.
Ward MK, Pollard WH. A hydrohalite spring deposit in the Canadian high Arctic: a potential Mars analogue. Earth Planet Sci Lett. 2018;504:126–38. https://doi.org/10.1016/j.epsl.2018.10.001.
Fateev EG. Anomalously low elastic stability of NaCl–H2O ice at low temperatures. Tech Phys. 2010;55:958–64. https://doi.org/10.1134/S1063784210070078.
Butler BM, Papadimitriou S, Day SJ, Kennedy H. Gypsum and hydrohalite dynamics in sea ice brines. Geochim Cosmochim Acta. 2017;213:17–34. https://doi.org/10.1016/j.gca.2017.06.020.
Steele-MacInnis M, Bodnar RJ. Effect of the vapor phase on the salinity of halite-bearing aqueous fluid inclusions estimated from the halite dissolution temperature. Geochim Cosmochim Acta. 2013;115:205–16. https://doi.org/10.1016/j.gca.2013.04.009.
Davila AF, Duport LG, Melchiorri R, Jänchen J, Valea S, de los Rios A, Fairén AG, Möhlmann D, McKay CP, Ascaso C, Wierzchos J. Hygroscopic salts and the potential for life on Mars. Astrobiology. 2010;10:617–28. https://doi.org/10.1089/ast.2009.0421.
Archer DG. Thermodynamic properties of the NaCl + H2O system. II. Thermodynamic properties of NaCl (aq), NaCl·2H2O (cr), and phase equilibria. J Phys Chem Ref Data. 1992;21:793–829. https://doi.org/10.1063/1.555915.
Drebushchak VA, Drebushchak TN, Ogienko AG, Yunoshev AS. Crystallization of sodium chloride dihydrate (hydrohalite). J Cryst Growth. 2019;517:17–23. https://doi.org/10.1016/j.jcrysgro.2019.04.009.
Pishchur DP, Drebushchak VA. Recommendations on DSC calibration: how to escape the transformation of a random error into the systematic error. J Therm Anal Calorim. 2016;124:951–8. https://doi.org/10.1007/s10973-015-5186-8.
Archer DG. Enthalpy increment measurements for NaCl (cr) and KBr (cr) from 4.5 K to 350 K, thermodynamic properties of the NaCl + H2O system. 3. J Chem Eng Data. 1997;42:281–92. https://doi.org/10.1021/je960224q.
Feistel R, Wagner W. A new equation of state for H2O ice Ih. J Phys Chem Ref Data. 2006;35:1021–47. https://doi.org/10.1063/1.2183324.
Pabalan RT, Pitzer KS. Thermodynamics of concentrated electrolyte mixtures and the prediction of mineral solubilities to high temperatures for mixtures in the system Na–K–Mg–Cl–SO4–OH–H2O. Geochim Cosmochim Acta. 1987;51:2429–43. https://doi.org/10.1016/0016-7037(87)90295-X.
Schlaikjer A, Thomsen K, Kontogeorgis GM. Simultaneous description of activity coefficients and solubility with eCPA. Ind Eng Chem Res. 2017;56:1074–89. https://doi.org/10.1021/acs.iecr.6b03333.
Young TF, Machin JS. Heat content and heat capacity of aqueous sodium chloride solutions. J Am Chem Soc. 1936;58:2254–60. https://doi.org/10.1021/ja01302a049.
Cohen-Adad R, Vallée P, Lorimer JW. Sodium chloride. In: Cohen-Adad R, Lorimer JW, editors. Alkali metal and ammonium chlorides in water and heavy water (binary systems), vol. 47., Solubility data seriesOxford: Pergamon Press; 1991. p. 64–209.
Mal’tseva NN, Khain VS. Borogidrid natriya (sodium borohydride). Moscow: Nauka; 1985.
Arkhangelskii IV, Tarasov VP, Kravchenko OV, Kirakosyan G, Tsvetkov MV, Solovev MV, Dobrovolskii YA, Shihovzev AV. Thermoanalytical and NMR investigation of NaBH4·2H2O thermolysis process. J Therm Anal Calorim. 2018;132:155–63. https://doi.org/10.1007/s10973-017-6821-3.
Sharma A, Tyagi VV, Chen CR, Buddhi D. Review on thermal energy storage with phase change materials and applications. Renew Sust Energy Rev. 2009;13:318–45. https://doi.org/10.1016/j.rser.2007.10.005.
Zeng JL, Shu L, Jiang LM, Chen YH, Zhang YX, Xie T, Sun LX, Cao Z. Thermodynamic and thermal energy storage properties of a new medium-temperature phase change material. J Therm Anal Calorim. 2019;135:3171–9. https://doi.org/10.1007/s10973-018-7530-2.
Guo L, Yu X, Gao D, Guo Y, Ma C, Deng T. Synthesis and thermal energy storage properties of a calcium-based room temperature phase change material for energy storage. J Therm Anal Calorim. 2019;135:3215–21. https://doi.org/10.1007/s10973-018-7610-3.
Xu X, Zhang X, Zhou S, Wang Y, Lu L. Experimental and application study of Na2SO4·10H2O with additives for cold storage. J Therm Anal Calorim. 2019;136:505–12. https://doi.org/10.1007/s10973-018-7633-9.
Lowitz TE. Observations on the crystallization of common salt under cooling and the new way of purification of the salt. Nova Acta Acad Sci. 1794;8:364–9 (in Latin).
Lowitz TE. Selected works on chemistry and chemical technology. Moscow: Publishing House of the USSR Academy of Sciences; 1955. p. 174–8 (Russian translation).
Acknowledgements
VAD acknowledges that his work was supported by state assignment Project No. 0330-2019-0004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drebushchak, V.A., Ogienko, A.G. Calorimetric measurements of sodium chloride dihydrate (hydrohalite). J Therm Anal Calorim 140, 2555–2562 (2020). https://doi.org/10.1007/s10973-019-08954-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08954-7