Abstract
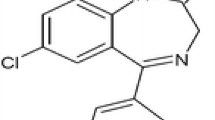
The derivative of 1,2,4-thiadiazole (TDZ) can be considered as a perspective agent for the Alzheimer’s disease prevention. Due to a highly lipophilic character, the compound reveals good membrane permeability properties and poor solubility in aqueous media. In order to solve this problem, water-soluble biodegradable polymers: PEG 6000 (PEG), PVP K29-30 (PVP) and linear (A–B–A type) ethylene oxide-propylene oxide block copolymer Pluronic F127 (F127) were used in the present investigation to obtain binary and ternary TDZ composites (solid dispersions) with improved solubility and dissolution rate. Composites were prepared by a mechanical grinding procedure. Differential scanning calorimetry and FTIR spectroscopy were used to characterize the obtained samples. The interaction of TDZ with PVP after the grinding procedure was revealed. Shake-flask method was applied to measure the solubility of the composites. A dramatical increase in the solubility was shown for the solid dispersion with F127 above the critical micelle concentration. The dissolution behavior was studied with the help of the basket method at pH 1.2 and pH 6.8. The dissolution of TDZ/polymer solid dispersions was substantially accelerated as compared to pure TDZ and physical mixtures. The dissolution mechanism was estimated through the Korsmeyer–Peppas model equation. Franz diffusion cell and new Permeapad™ barrier were applied for the permeability assay. The permeability of TDZ in solid dispersions through the Permeapad™ barrier was shown to decrease in comparison with the pure TDZ. It was concluded that composites with PEG, PVP and F127 are an effective tool for increasing the solubility and dissolution of 1,2,4-thiadiazole derivative.







Similar content being viewed by others
References
Shekhawat P, Pokharkar V. Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm Sin B. 2017;7(3):260–80.
Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3:631–43.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53.
Basavaraj S, Betageri GV. Can formulation and drug delivery reduce attrition during drug discovery and development—review of feasibility, benefits and challenges. Acta Pharm Sin B. 2014;4(1):3–17.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–96.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–30.
Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3):799–813.
Huang Y, Dai W-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4(1):18–25.
Bruni G, Sakaj M, Berbenni V, Maggi L, Friuli V, Girella A, Chiara Milanese Marini A. Physico-chemical and pharmaceutical characterization of sulindac–proglumide binary system. J Therm Anal Calorim. 2019;136:2063–70.
Elder DP, Holm R, De Diego HL. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int J Pharm. 2013;453(1):88–100.
Trandafirescu C, Ledeţi I, Şoica C, Ledeţi A, Vlase G, Borcan F, Dehelean C, Coricovac D, Racoviceanu R, Aigner Z. Albendazole-cyclodextrins binary systems. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08326-1.
Semalty A. Cyclodextrin and phospholipid complexation in solubility and dissolution enhancement: a critical and meta-analysis. Exp Opin Drug Deliv. 2014;11(8):1255–72.
Vieira ACC, Ferreira Fontes DA, Chaves LL, Alves LDS, de Freitas Neto JL, de La Roca Soares MF, Rolim-Neto PJ. Multicomponent systems with cyclodextrins and hydrophilic polymers for the delivery of Efavirenz. Carbohyd Polym. 2015;130:133–40.
Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50.
Pires FQ, Pinho LA, Freire DO, Silva ICR, Sa-Barreto LL, Cardozo-Filho L, Gratieri T, Gelfuso GM, Cunha-Filho M. Thermal analysis used to guide the production of thymol and Lippia origanoides essential oil inclusion complexes with cyclodextrin. J Therm Anal Calorim. 2019;137(2):543–53.
Pandya P, Gattani S, Jain P, Khirwal L, Kljgh SS. Co-solvent evaporation method for enhancement of solubility and dissolution rate of poorly aqueous soluble drug simvastatin: in vitro–in vivo evaluation. AAPS PharmSciTech. 2008;9(4):1247–52.
Gharee MM, Abdulrasool AA, Hussein AA, Noordin MI. Kneading technique for preparation of binary solid dispersion of meloxicam with poloxamer 188. AAPS PharmSciTech. 2009;10(4):1206–15.
Majerik V, Horváth G, Szokonya L, Charbit G, Badens E, Bosc N, Teillaud E. Supercritical antisolvent versus coevaporation: preparation and characterization of solid dispersions. Drug Dev Ind Pharm. 2007;33(9):975–83.
Yang C, Xu X, Wang J, An Z. Use of the co-grinding method to enhance the dissolution behavior of a poorly water-soluble drug: generation of solvent-free drug-polymer solid dispersions. Chem Pharm Bull (Tokyo). 2012;60(7):837–45.
Kanaze FI, Kokkalou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: a comparative study. J Appl Polym Sci. 2006;102:460–71.
Patel RP, Patel DJ, Bhimani DB, Patel JK. Physicochemical characterization and dissolution study of solid dispersions of furosemide with polyethylene glycol 6000 and Polyvinylpyrrolidone K30. Diss Tech. 2008;17–25.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Bailey Jr, Frederick E, Koleske JV. Alkylene oxides and their polymers. 1990; New York: Dekker. pp. 27–28. ISBN 9780824783846. Retrieved 17 July 2017.
Cavallaro G, Grillo I, Gradzielski M, Lazzara G. Structure of hybrid materials based on halloysite nanotubes filled with anionic surfactants. J Phys Chem C. 2016;120(25):13492–502.
Dintcheva N, Catalano G, Arrigo R, Morici E, Cavallaro G, Lazzara G, Bruno M. Pluronic nanoparticles as anti-oxidant carriers for polymers. Polym Degrad Stab. 2016;134:194–201.
Karolewicz B, Gajda M, Górniak A, Owczarek A, Mucha I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J Therm Anal Calorim. 2017;130:383–90.
Chakraborti CK, Sahoo S, Behera PK. Role of different biodegradable polymers on the permeability of ciprofloxacin. J Adv Pharm Technol Res. 2014;5(3):140–7.
Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, Amidon GL, Dahan A. The solubility-permeability interplay: mechanistic modeling and predictive application of the impact of micellar solubilization on intestinal permeation. Mol Pharm. 2011;8:1848–56.
Beig A, Miller JM, Dahan A. Accounting for the solubility–permeability interplay in oral formulation development for poor water solubility drugs: the effect of PEG-400 on carbamazepine absorption. Eur J Pharm Biopharm. 2012;81:386–91.
Miller JM, Beig A, Carr RA, Webster GK, Dahan A. The solubility—permeability interplay when using cosolvents for solubilization: revising the way we use solubility-enabling formulations. Mol Pharm. 2012;9:581–90.
Siddiquia N, Ahuja P, Ahsan W, Pandey SN, Alam MS. Thiadiazoles: progress report on biological activities. J Chem Pharm Res. 2009;1(1):19–30.
MacLeod AM, Baker R, Freedman SB, Patel S, Merchant KJ, Roe M, Saunders J. Synthesis and muscarinic Activities of 1,2,4-Thiadiazoles. J Med Chem. 1990;33:2052–9.
Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First Non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45:1292–9.
Juszczak M, Walczak K, Langner E, Karpinska M, Matysiak J, Rzeski W. Neuroprotective activity of 2-amino-1,3,4-thiadiazole derivative 4BrABT—an in vitro study. Ann Agr Env Med. 2013;20:575–9.
Castano T, Encinas A, Peґrez C, Castro A, Campillo NE, Gil C. Design, synthesis, and evaluation of potential inhibitors of nitric oxide synthase. Bioorg Med Chem. 2008;16:6193–206.
Volkova TV, Terekhova IV, Silyukov OI, Proshin AN, Bauer-Brandl A, Perlovich GL. Towards the rational design of novel drugs based on solubility, partitioning/distribution, biomimetic permeability and biological activity exemplified by 1,2,4-thiadiazole derivatives. Med Chem Commun. 2017;8:162–75.
di Cagno M, Bibi HA, Bauer-Brandl A. New biomimetic barrier Permeapad™ for efficient investigation of passive permeability of drugs. Eur J Pharm Sci. 2015;73:29–34.
Lin HL, Lin S-Y, Lin C-C, Hsu C-H, Wu T-K, Huang Y-T. Mechanical grinding effect on thermodynamics and inclusion efficiency of loratadine-cyclodextrin inclusion complex formation. Carbohydr Polym. 2012;87:512–7.
Rojas-Oviedo I, Retchkiman-Corona B, Quirino-Barreda CT, Cárdenas J, Schabes-Retchkiman PS. Solubility enhancement of a poorly water soluble drug by forming solid dispersions using mechanochemical activation. Indian J Pharm Sci. 2012;74(6):505–11.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem and Instr. 1965;4:117–212.
Bibi HA, di Cagno M, Holm R, Bauer-Brandl A. Permeapad™ for investigation of passive drug permeability: the effect of surfactants, co-solvents and simulated intestinal fluids (FaSSIF and FeSSIF). Int J Pharm. 2015;493:192–7.
Munson EJ. Analytical Techniques in Solid-state Characterization. In: Qiu Y, Chen Y, Zhang GGZ, Liu L, Porter WR, editors. Developing Solid Oral Dosage Forms. Pharmaceutical Theory and Practice. Edinburgh: Academic Press; 2009. p. 61–74.
Jayaramudu T, Raghavendra GM, Varaprasad K, Reddy GVS, Reddy AB, Sudhakar K, Sadiku ER. Preparation and characterization of poly(ethyleneglycol) stabilized nanosilver particles by a mechanochemical assisted ball mill process. J Appl Polym Sci. 2016;133:43027–35.
Arias MJ, Moyano JR, Ginés JM. Study by DSC and HSM of the oxazepam-PEG 6000 and oxazepam-d-mannitol systems: application to the preparation of solid dispersions. Thermochim Acta. 1998;321:33–41.
Wang X, Michoel A, Van den Mooter G. Study of the phase behavior of polyethylene glycol 6000–itraconazole solid dispersions using DSC. Int J Pharm. 2004;272:181–7.
Verheyen S, Augustijns P, Kinget R, Van den Mooter G. Melting behavior of pure polyethyleneglycol 6000 and polyethyleneglycol 6000 in solid dispersions containing diazepam or temazepam: a DSC study. Thermochim Acta. 2001;380:153–64.
Karolewicz B, Gajda M, Pluta J, Gorniak A. Dissolution study and thermal analysis of fenofibrate–Pluronic F127 solid dispersions. J Therm Anal Calorim. 2015;125(2):751–7.
Karolewicz B, Gorniak A, Owczarek A, Zurawska-Płaksej E, Piwowar A, Pluta J. Thermal, spectroscopic, and dissolution studies of ketoconazole–Pluronic F127 system. J Therm Anal Calorim. 2014;115(3):2487–93.
Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, Higaki K, Kimura T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267:79–91.
Ginés JM, Arias MJ, Rabasco AM, Sánchez-Soto PJ. Thermal study of the system polyethyleneglycol 6000-triamterene. J Therm Anal. 1993;40:453–62.
Sezgin Z, Yuksel N, Baykara T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur J Pharm Biopharm. 2006;64:261–8.
Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly(ethy1ene oxide)-poly(propy1ene oxide)-poly(ethy1ene oxide) triblock copolymers in aqueous aolutions: thermodynamics of copolymer association. Macromolecules. 1994;27:2414–25.
Croy SR, Kwon GS. The effects of Pluronic block copolymers on the aggregation state of nystatin. J Control Release. 2004;95:161–71.
Cirri M, Mura P, Rabasco AM, Gines JM, Moyano JR, Gonzalez-Rodrıguez ML. Characterization of ibuproxam binary and ternary dispersions with hydrophilic carriers. Drug Dev and Ind Pharm. 2004;30:65–74.
Chadha R, Kapoor KV, Kumar A. Analytical techniques used to characterize drug-polyvinylpyrrolidone systems in solid and liquid states—An overview. JSIR. 2006;65:459–69.
Zhang H, Yu L. Dissolution Testing for Solid Oral Drug Products: theoretical Considerations. Am Pharm Rev. 2004;7:26–31.
Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27(1):48–9.
Reflection paper on the dissolution specification for generic oral immediate release products. European Medicines Agency, 2016.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Singhvi G, Singh M. Review: in vitro drug release characterization models. IJPSR. 2011;II(I):77–84.
Brusnikina M, Silyukov O, Chislov M, Volkova T, Proshin A, Terekhova I. New water-soluble dosage forms of 1,2,4-thiadiazole derivative on the basis of inclusion complexes with cyclodextrins. J Therm Anal Calorim. 2017;127:1815–24.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Sinclair GW, Peppas NA. Analysis of non-Fickian transport in polymers using a simplified exponential expression. J Membr Sci. 1984;17:329–31.
Hezaveh S, Samanta S, De Nicola A, Milano G, Roccatano D. Understanding the interaction of block copolymers with DMPC lipid bilayer using coarse-grained molecular dynamics simulations. J Phys Chem B. 2012;116(49):14333–45.
Nawaz S, Redhead M, Mantovani G, Alexander C, Bosquillon C, Carbone P. Interactions of PEO–PPO–PEO block copolymers with lipid membranes: a computational and experimental study linking membrane dialysis with polymer structure. Soft Matter. 2012;8:6744–54.
Acknowledgements
This investigation was performed within the State Program of Fundamental Scientific Research (No. 0092-2014-0005). The authors thank Prof. Annette Bauer-Brandl (Dept. of Physics Chemistry and Pharmacy, University of Southern Denmark) and Labtastic distributor (https://labtastic.shop) for donation of the Permeapad™ barrier. Authors thank the “The Upper Volga Region Centre of Physicochemical Research” (Ivanovo, Russia) and St. Petersburg State University Center “Thermogravimetric and Calorimetric Research” for the provision of scientific equipment (DSC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Volkova, T.V., Domanina, E.N., Chislov, M.V. et al. Polymeric composites of 1,2,4-thiadiazole: solubility, dissolution and permeability assay. J Therm Anal Calorim 140, 2305–2315 (2020). https://doi.org/10.1007/s10973-019-08947-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08947-6